Abstract
The influence of pH of the gelatin solution on the cycle performance of the sulfur cathode for lithium–sulfur batteries was studied. Scanning electron microscopy measurements revealed that the cathode prepared with pH 10.0 gelatin solution as a binder showed a uniform distribution. The charge/discharge experiments of the lithium–sulfur batteries showed that an initial capacity of  and a reversible capacity of
and a reversible capacity of  after 30 cycles were achieved, which were higher than those of the cathodes prepared at pH 5.0 and 8.0. To understand the effect of the pH value of the gelatin solution on the cycle performance of sulfur cathodes, electrochemical impedance spectroscopy and X-ray diffractometry were carried out.
after 30 cycles were achieved, which were higher than those of the cathodes prepared at pH 5.0 and 8.0. To understand the effect of the pH value of the gelatin solution on the cycle performance of sulfur cathodes, electrochemical impedance spectroscopy and X-ray diffractometry were carried out.
Export citation and abstract BibTeX RIS
There is an ever increasing demand for the development of rechargeable lithium batteries with high energy density and long cycle life suitable for a variety of applications ranging from microbatteries of miniature electronic devices to large power sources of electric vehicles.1–4 Elemental sulfur is an active mass which has a high theoretical specific capacity of  , assuming the complete reaction of lithium with sulfur to
, assuming the complete reaction of lithium with sulfur to  . Moreover, many advantages such as low cost, abundance in nature, and environment friendliness make the lithium–sulfur battery a great potential for future use, thereby attracting considerable interests.5–7 However, lithium–sulfur batteries have critical drawbacks in the aspect of cycle life, rate capability, and sulfur utilization.8, 9 There were many attempts to overcome these problems, 10–16 such as optimization of the organic electrolyte and room-temperature ionic liquid, sulfur–high porous carbon composite, and sulfur-coated multiwalled carbon nanotube composite. Some interesting cathode developments involving sulfides have been reported recently: highly ordered interwoven C–S composites were created and its polymer modification reached reversible capacities up to
. Moreover, many advantages such as low cost, abundance in nature, and environment friendliness make the lithium–sulfur battery a great potential for future use, thereby attracting considerable interests.5–7 However, lithium–sulfur batteries have critical drawbacks in the aspect of cycle life, rate capability, and sulfur utilization.8, 9 There were many attempts to overcome these problems, 10–16 such as optimization of the organic electrolyte and room-temperature ionic liquid, sulfur–high porous carbon composite, and sulfur-coated multiwalled carbon nanotube composite. Some interesting cathode developments involving sulfides have been reported recently: highly ordered interwoven C–S composites were created and its polymer modification reached reversible capacities up to  .17 Besides, the investigation on the cathode binders of lithium–sulfur batteries also showed improvement.18 Jung and Kim improved the cycle performance of lithium–sulfur batteries by using a mixed binder system of poly(4-vinyl)pyridine and poly(ethyleneimine), which can maintain the morphology of the cathode during charge–discharge cycles.19
.17 Besides, the investigation on the cathode binders of lithium–sulfur batteries also showed improvement.18 Jung and Kim improved the cycle performance of lithium–sulfur batteries by using a mixed binder system of poly(4-vinyl)pyridine and poly(ethyleneimine), which can maintain the morphology of the cathode during charge–discharge cycles.19
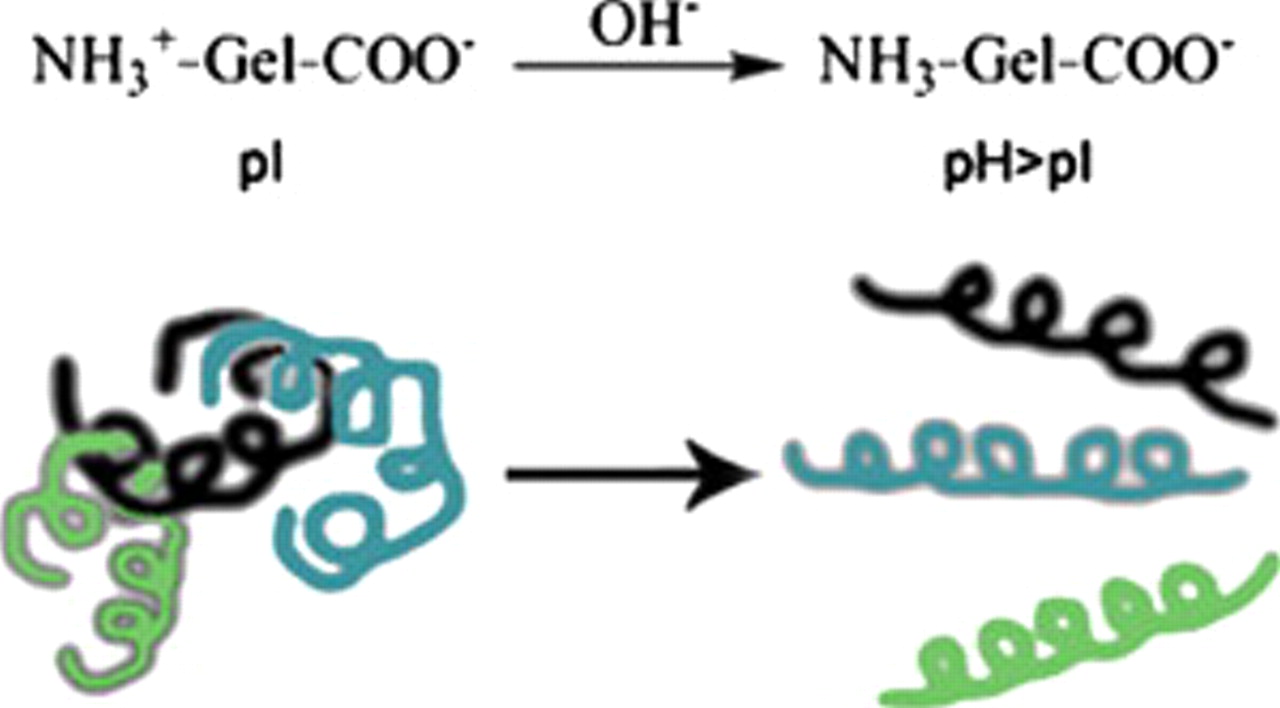
In our previous work, we found that the gelatin had multifunctional effects on the sulfur cathode in the lithium–sulfur battery: It was not only a high adhesion agent and a strong dispersion agent for the cathode materials but also an electrochemically stable binder. Our research showed that it is more suitable for use in lithium–sulfur batteries than some conventional binders, such as poly(vinylidene fluoride) and poly(ethylene oxide).20–23 Gelatin contains both acidic carboxyl and basic amino groups, which can transform into the corresponding negatively and positively charged groups in solution.24–26 When the pH value of the gelatin solution is at its isoelectric point (IEP), it shows the lowest viscosity. This can be attributed to the molecular structures that appear like clews (as shown in Fig. 1). While the pH value is higher than the pH value at IEP (PI), gelatin can be considered as an "anionic" polymer with a large amount of –COO– groups. Due to electrostatic repulsion, the gelatin molecule stretches, and as the viscosity of the solution increases, the dispersion of the gelatin becomes better. In this paper, the effect of the pH value of the gelatin solution on the sulfur cathode in lithium–sulfur batteries was investigated. The structure and the electrochemical performances of the sulfur cathode were measured by scanning electron microscopy (SEM), X-ray diffractometry (XRD), electrochemical impedance spectroscopy (EIS), and charge–discharge experiments.
Figure 1. The conformation change in gelatin molecule with different pH values.
Experimental
Preparation of the cathode
The gelatin (type B, derived from pig bone, its IEP is about 5.2) solution used was prepared by dissolving gelatin in deionized water under  . The pH value was adjusted by 0.1 M lithium hydroxide and hydrochloric acid, respectively. The viscosity of the solution was measured by a Ubisch viscometer.
. The pH value was adjusted by 0.1 M lithium hydroxide and hydrochloric acid, respectively. The viscosity of the solution was measured by a Ubisch viscometer.
Elemental sulfur (99.5%, analytical grade, Beijing, China) and acetylene black (AB, Jinpu. Corp., China) were dried at  for 5 h under vacuum before use. The gelatin solution was used as a binder for the sulfur cathode. The cathode slurry was prepared by milling at room temperature and then coated onto Al foil by a doctor blade. Both cathodes were dried under vacuum (vacuum degree: −0.1 Mpa) at
for 5 h under vacuum before use. The gelatin solution was used as a binder for the sulfur cathode. The cathode slurry was prepared by milling at room temperature and then coated onto Al foil by a doctor blade. Both cathodes were dried under vacuum (vacuum degree: −0.1 Mpa) at  for 10 h, then cut into a small square with an area of
for 10 h, then cut into a small square with an area of 
 . The compositions of the cathodes were 63 wt % sulfur, 7 wt % gelatin, and 30 wt % AB.
. The compositions of the cathodes were 63 wt % sulfur, 7 wt % gelatin, and 30 wt % AB.
These sulfur electrodes were assembled into coin-type cells in a glove box, with lithium foils as anodes and 1 M  dimethoxyethane/(1,3-dioxolane)
dimethoxyethane/(1,3-dioxolane)  (received from Beijing Chemical Reagent Research Institute) as the electrolyte.
(received from Beijing Chemical Reagent Research Institute) as the electrolyte.
Electrochemical property measurements
The discharge and charge capacities of the cells were measured galvanostatically at a discharge current density of  and a charge current density of
and a charge current density of  between 1.5 and 2.8 V by a cell test instrument (LAND Electronic Co. CT2001A) at room temperature. The morphology changes in the sulfur cathodes and the redox process were examined using a scanning electron microscope (Hitachi S-4700) and X-ray diffractometer (D/max 2500 V, Rigaku, scintillation counter probe SC-70, normal holder) at 0.5 mV/s. The electrochemical impedance spectra were measured by PARSTAT 2273.
between 1.5 and 2.8 V by a cell test instrument (LAND Electronic Co. CT2001A) at room temperature. The morphology changes in the sulfur cathodes and the redox process were examined using a scanning electron microscope (Hitachi S-4700) and X-ray diffractometer (D/max 2500 V, Rigaku, scintillation counter probe SC-70, normal holder) at 0.5 mV/s. The electrochemical impedance spectra were measured by PARSTAT 2273.
Results and Discussion
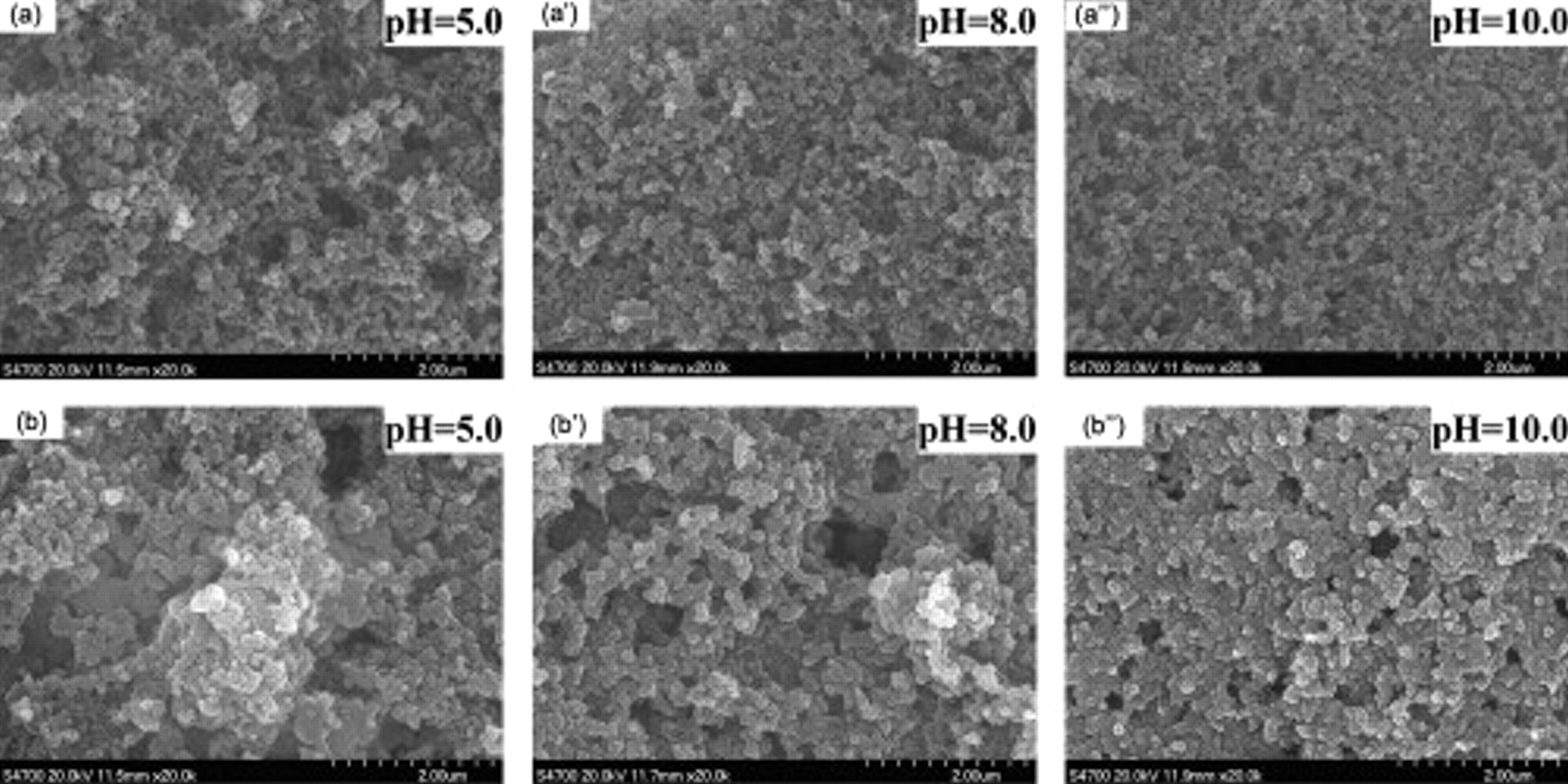
Figure 2a , 2a', and 2a'' shows the SEM images of the sulfur cathodes with the gelatin binder prepared at different pH values. Clearly, the surface morphologies of these cathodes are dissimilar. The cathode prepared at pH 10.0 showed the best dispersion of the powders. Moreover, after the first discharge process, it is clear that at pH 10.0, the cathode dispersed more uniformly during the discharge process, which would be helpful in improving the capacity and cycle stability of the battery. The reason is that at pH 10.0, far from the IEP, the gelatin molecule in the solution stretches and the materials diffused in the solution tend to be dispersed uniformly because of its conformation in the solution (as shown in Fig. 1). The uniform dispersion of the cathode benefits the electrochemical performance of the battery, thus the result shows that the pH value far from the IEP could be selected.
Figure 2. SEM images of sulfur cathodes with gelatin binder prepared at different pH values [(a),  , and
, and  ] before discharge and [(b),
] before discharge and [(b),  , and
, and  ] after the first discharge.
] after the first discharge.
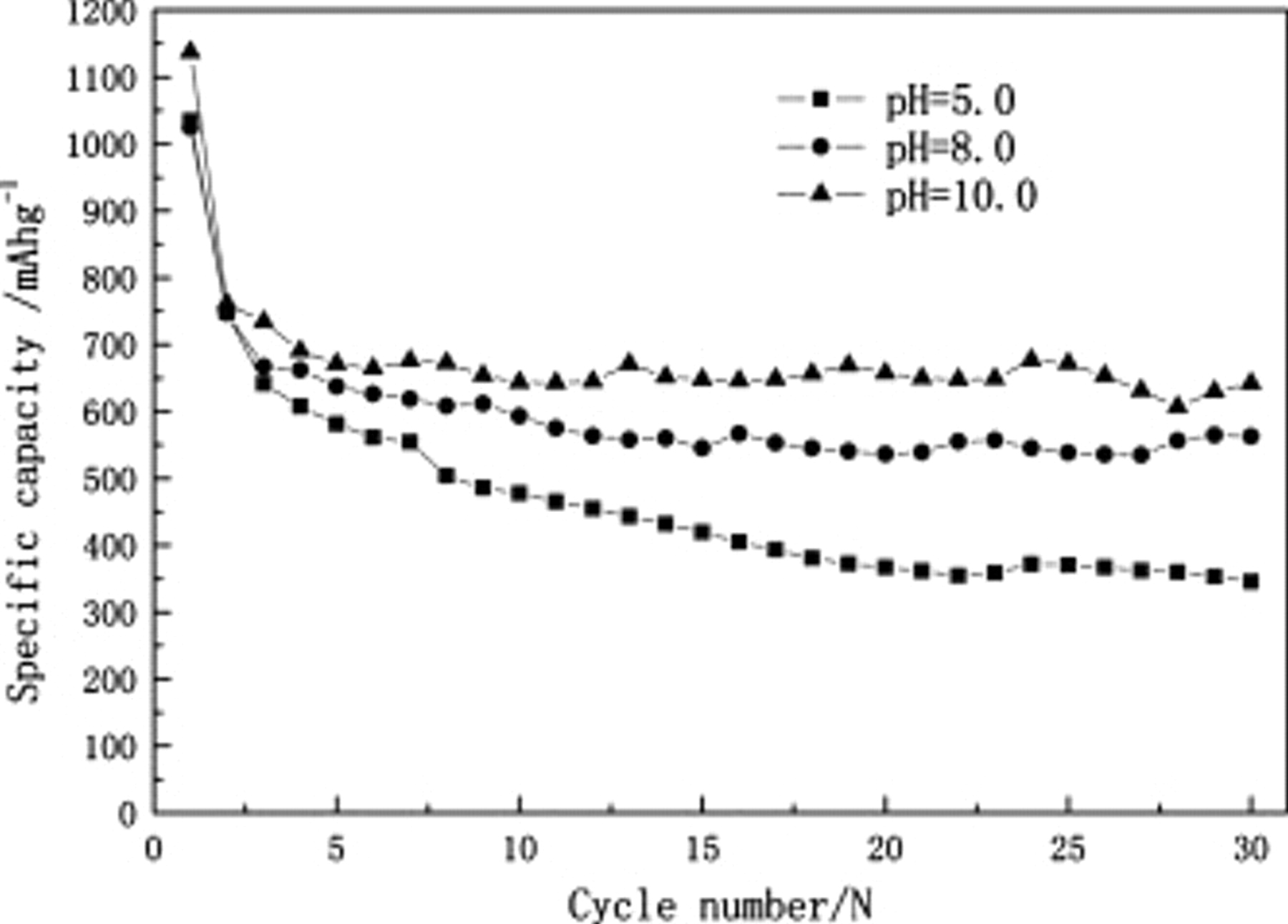
The cycle performances of these three cathodes are presented in Fig. 3. The cathode prepared at pH 10.0 showed a high initial discharge capacity of  , which is higher than the data obtained from the other two cathodes prepared at pH 8.0
, which is higher than the data obtained from the other two cathodes prepared at pH 8.0  and pH 5.0
and pH 5.0  . Moreover, the capacity fading rate of the cathode obtained from the pH 10.0 solution is the lowest. The discharge capacity of the cathode prepared at pH 10.0 remains at
. Moreover, the capacity fading rate of the cathode obtained from the pH 10.0 solution is the lowest. The discharge capacity of the cathode prepared at pH 10.0 remains at  after 30 cycles, which is much higher than those of the cathodes prepared at pH 8.0
after 30 cycles, which is much higher than those of the cathodes prepared at pH 8.0  and pH 5.0
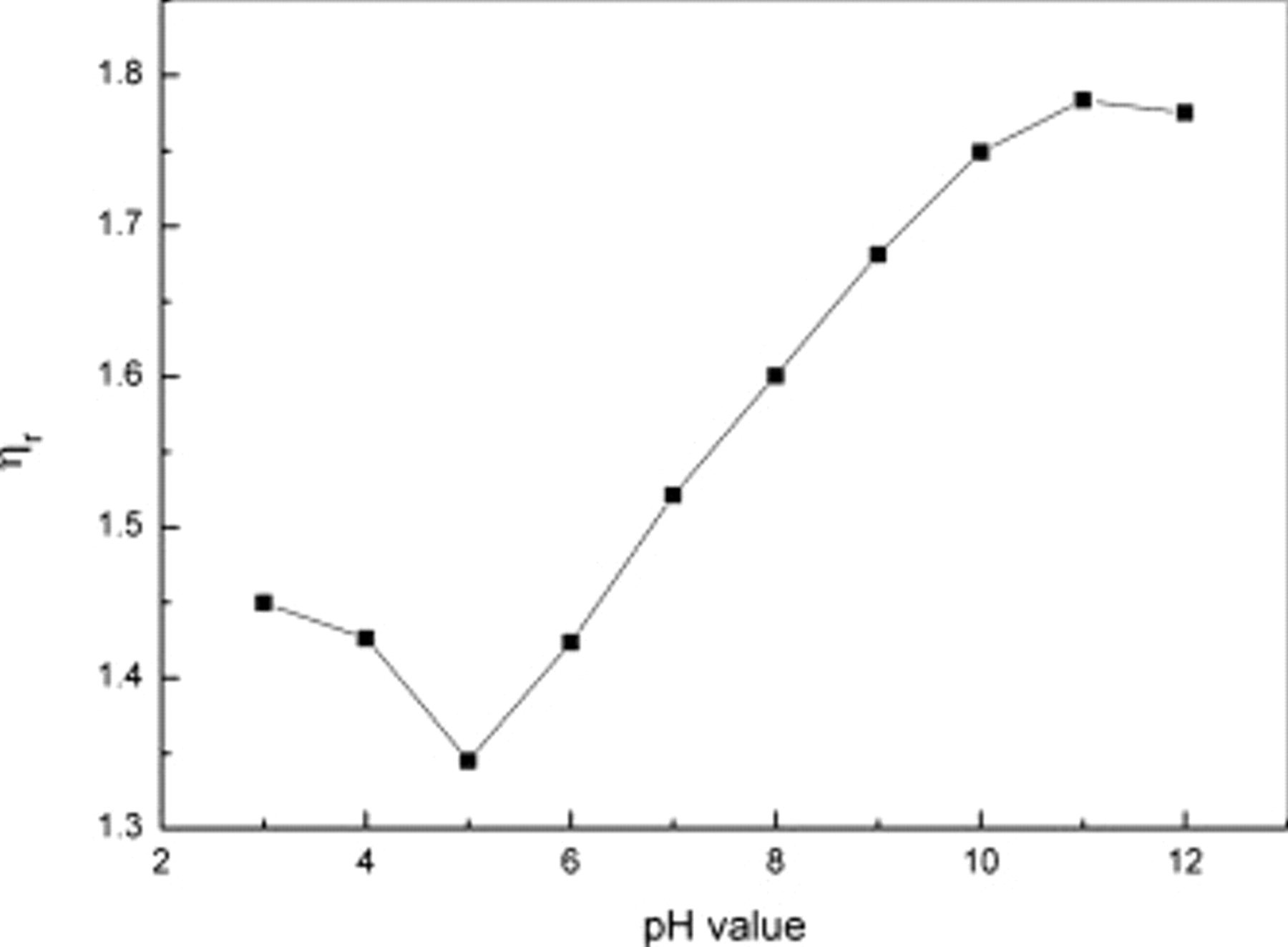
and pH 5.0  , and these results are consistent with the conclusion forecasted through the SEM images shown in Fig. 2a, 2a', and 2a''. This can be explained by the viscosity of the gelatin and the dispersion of the cathode. The gelatin solution at pH 10.0 has a higher viscosity than at pH 8.0 and 5.0 (as shown in Fig. 4), which would be helpful in maintaining the cathode morphology, as shown in Fig. 2. The different viscosity of the gelatin solution and molecular conformation in the gelatin solution caused by the pH value of the gelatin solution might lead to some differences in the electrochemical performance of the cathodes that used the gelatin as binders. From the discussion above, it is believed that when the pH value of the gelatin solution was increased from pH 5.0 to 10.0, the structure of the cathode became more stable and the cycle performance of the battery became better. That might be because at pH 10.0, the viscosity of the gelatin was high and the gelatin molecule stretched in the solution which could have helped the cathode materials gather more stability and slowed down the dissolution of polysulfide, thereby resulting in a longer life and a high capacity. Moreover, the good dispersion of active materials in the cathode prepared at pH 10.0 may have caused the excellent electric conductivity, making it beneficial to the redox during the charge–discharge process.
, and these results are consistent with the conclusion forecasted through the SEM images shown in Fig. 2a, 2a', and 2a''. This can be explained by the viscosity of the gelatin and the dispersion of the cathode. The gelatin solution at pH 10.0 has a higher viscosity than at pH 8.0 and 5.0 (as shown in Fig. 4), which would be helpful in maintaining the cathode morphology, as shown in Fig. 2. The different viscosity of the gelatin solution and molecular conformation in the gelatin solution caused by the pH value of the gelatin solution might lead to some differences in the electrochemical performance of the cathodes that used the gelatin as binders. From the discussion above, it is believed that when the pH value of the gelatin solution was increased from pH 5.0 to 10.0, the structure of the cathode became more stable and the cycle performance of the battery became better. That might be because at pH 10.0, the viscosity of the gelatin was high and the gelatin molecule stretched in the solution which could have helped the cathode materials gather more stability and slowed down the dissolution of polysulfide, thereby resulting in a longer life and a high capacity. Moreover, the good dispersion of active materials in the cathode prepared at pH 10.0 may have caused the excellent electric conductivity, making it beneficial to the redox during the charge–discharge process.
Figure 3. Cycle performance of lithium/sulfur cells at a current density of  .
.
Figure 4. Correlation between the pH value and the viscosity  .
.
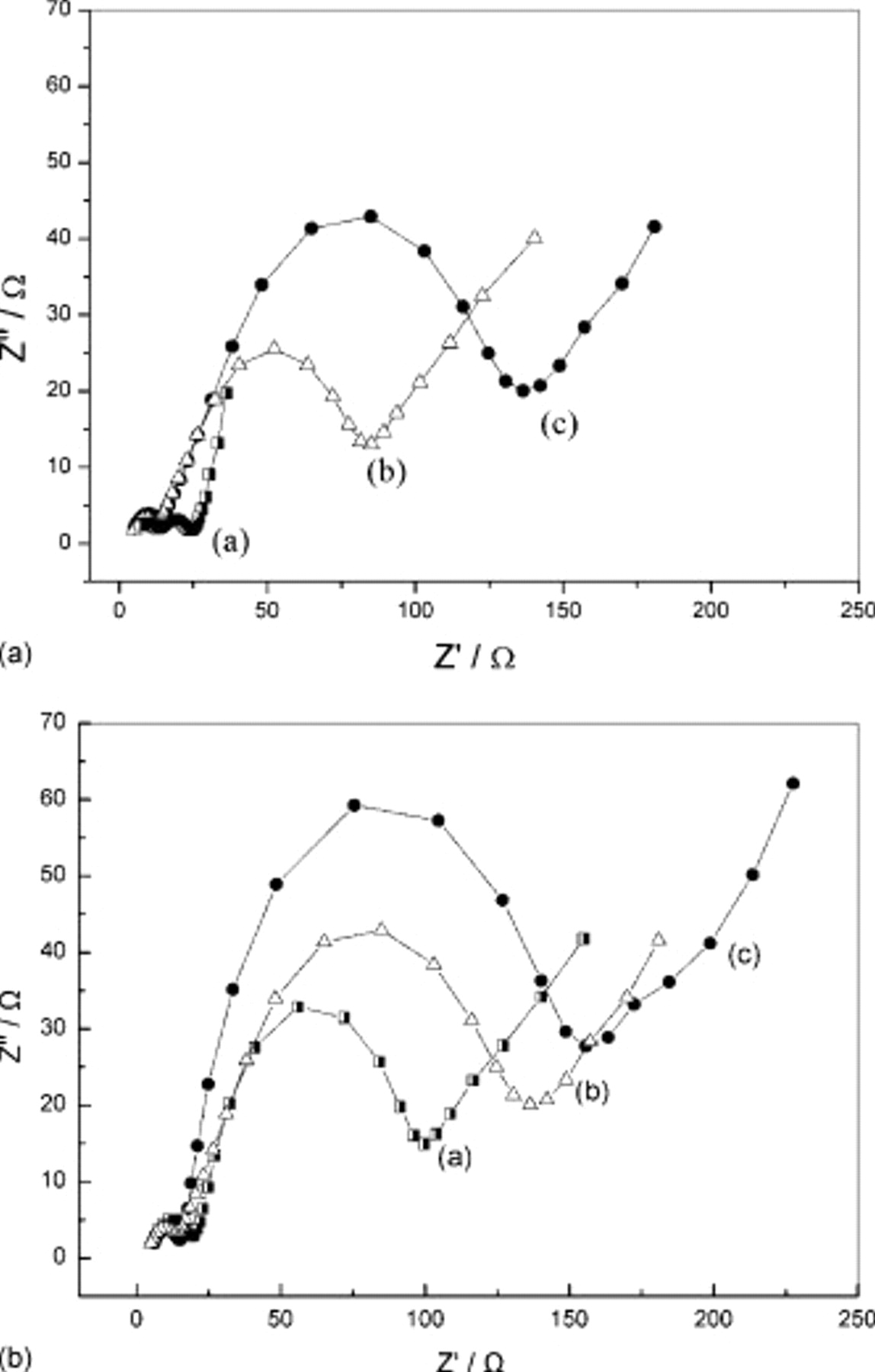
The EIS measurements (three electrodes) of the three cathodes are shown in Fig. 5, and we set the rest period at 2 h. At high frequencies, the impedance spectrum exhibits a semicircular loop. The diameter of this semicircle indicates contact resistance and charge-transfer resistance. As the pH value of the solution increased, the impedance of the cathode decreased. The cathode prepared with pH 10.0 gelatin solution as a binder displayed a much lower charge-transfer resistance than that of the cathodes obtained with the other two pH conditions. Moreover, after the first discharge process, the charge-transfer resistance showed the same trend as that before the discharge (as shown in Fig. 5b). This may be explained by the sulfur dispersion on the cathode. The uniformly dispersed sulfur is beneficial to the electron and charge transfer on the electrode surface. In particular, the highly dispersed insulating sulfur on the electrically conductive matrix decreases the diffusion path of the lithium ions in the cathode.
Figure 5. Electrochemical impedance spectra of lithium/sulfur cells with gelatin binders prepared at different pH conditions: (a) pH 10.0, (b) 8.0, and (c) 5.0. (a) Before discharge and (b) after one cycle.
The changes in the XRD patterns as a function of different charge–discharge states are shown in Fig. 6. From Fig. 6a and a', the orthorhombic crystal structure of elemental sulfur can be seen, which indicates that no new phase is formed during the preparation of the cathode. The peak area of sulfur decreased or even disappeared in Fig. 6 and b', and the diffraction peaks coincided with  , appearing after the first discharge process. However, the intensities of the peaks are different. The intensities of sulfur before discharge (Fig. 6a and a') and after the first discharge (Fig. 6b and 6b') are almost the same, but through our approximate calculation on the sulfur and
, appearing after the first discharge process. However, the intensities of the peaks are different. The intensities of sulfur before discharge (Fig. 6a and a') and after the first discharge (Fig. 6b and 6b') are almost the same, but through our approximate calculation on the sulfur and  peaks, we found that after one charge process, the area of sulfur at pH 10.0 is obviously greater than that at the pH 5.0 cathode. The decreased rate of the pH 10.0 cathode was only 0.4%, which is smaller than the data of pH 5.0 (nearly 90%). Moreover, both intensities of the
peaks, we found that after one charge process, the area of sulfur at pH 10.0 is obviously greater than that at the pH 5.0 cathode. The decreased rate of the pH 10.0 cathode was only 0.4%, which is smaller than the data of pH 5.0 (nearly 90%). Moreover, both intensities of the  peaks and the elemental sulfur peaks of the pH 10.0 cathode are the strongest, which indicates that the redox reaction of this cathode is the most complete. Thus, it leads to the highest initial discharge capacity and charge capacity. Therefore, comparing the two images, we can clearly see that the alkaline condition of the gelatin solution (pH 10.0) benefited the redox reaction of the battery. At pH 10.0, the viscosity of gelatin is higher than at pH 5.0 and the molecule is stretched, which means that the high viscosity of the gelatin solution could not only tightly conglutinate the materials on the surface of the cathode but also make the redox process more complete. Therefore, it is believed that previous results reveal that the pH condition affects the redox reaction of the sulfur cathode. Because its viscosity becomes higher with an increasing pH value ranging from 5.0 to 10.0, the gelatin adhered more stably to the cathode materials and slowed down the dissolution of polysulfide, thereby extending the lower plateau region and increasing the capacity.
peaks and the elemental sulfur peaks of the pH 10.0 cathode are the strongest, which indicates that the redox reaction of this cathode is the most complete. Thus, it leads to the highest initial discharge capacity and charge capacity. Therefore, comparing the two images, we can clearly see that the alkaline condition of the gelatin solution (pH 10.0) benefited the redox reaction of the battery. At pH 10.0, the viscosity of gelatin is higher than at pH 5.0 and the molecule is stretched, which means that the high viscosity of the gelatin solution could not only tightly conglutinate the materials on the surface of the cathode but also make the redox process more complete. Therefore, it is believed that previous results reveal that the pH condition affects the redox reaction of the sulfur cathode. Because its viscosity becomes higher with an increasing pH value ranging from 5.0 to 10.0, the gelatin adhered more stably to the cathode materials and slowed down the dissolution of polysulfide, thereby extending the lower plateau region and increasing the capacity.
Figure 6. XRD patterns of sulfur cathodes during charge–discharge  process: [(a) and
process: [(a) and  ] Original, [(b) and
] Original, [(b) and  ] first discharge, and [(c) and
] first discharge, and [(c) and  ] first charge.
] first charge.
Conclusion
The pH value of the gelatin solution as a binder affected the cycle performance of the sulfur cathode in lithium–sulfur batteries. A different pH value results in a different conformation of the gelatin molecule. When the pH value is far from the IEP, the gelatin molecular conformation in the solution tends to make the diffusion more uniform than at a point near the IEP. Thus, compared with pH 8.0 and 5.0, at pH 10.0, the dispersion of the active material in the cathode is more symmetrical, the initial discharge capacity of the cathode is higher, and the cycle performance is better. Moreover, the structure of the cathode prepared at pH 10.0 is more stable after the cycles. The cathode prepared with pH 10.0 gelatin binder solution achieved the highest initial capacity of  , which is better than cathodes prepared at pH 5.0 and 8.0. Moreover, it achieved the highest reversible capacity of
, which is better than cathodes prepared at pH 5.0 and 8.0. Moreover, it achieved the highest reversible capacity of  after 30 cycles.
after 30 cycles.
Beijing University of Chemical Technology assisted in meeting the publication costs of this article.