Abstract
The evolution of the Mn charge state, chemical composition, and electronic structure of  (LSMO) cathodes during the catalytic activation of solid oxide fuel cell (SOFC) has been studies using X-ray spectroscopy of as-processed, exposed, and activated dense thin LSMO films. Comparison of O
(LSMO) cathodes during the catalytic activation of solid oxide fuel cell (SOFC) has been studies using X-ray spectroscopy of as-processed, exposed, and activated dense thin LSMO films. Comparison of O  -edge and Mn
-edge and Mn  -edge X-ray absorption spectra from the different stages of LSMO cathodes revealed that the largest change after the activation occurred in the Mn charge state with little change in the oxygen environment. Core-level X-ray photoemission spectroscopy and Mn
-edge X-ray absorption spectra from the different stages of LSMO cathodes revealed that the largest change after the activation occurred in the Mn charge state with little change in the oxygen environment. Core-level X-ray photoemission spectroscopy and Mn  resonant photoemission spectroscopy studies of exposed and as-processed LSMO determined that the SOFC environment (
resonant photoemission spectroscopy studies of exposed and as-processed LSMO determined that the SOFC environment ( ambient pressure of
ambient pressure of  ) alone results in La deficiency (severest near the surface with Sr doping
) alone results in La deficiency (severest near the surface with Sr doping  ) and a stronger
) and a stronger  contribution, leading to the increased insulating character of the cathode prior to activation. Meanwhile, O
contribution, leading to the increased insulating character of the cathode prior to activation. Meanwhile, O  -edge X-ray absorption measurements support
-edge X-ray absorption measurements support  enrichment nearer the surface, along with the formation of mixed
enrichment nearer the surface, along with the formation of mixed  and/or passive
and/or passive  and SrO species.
and SrO species.
Export citation and abstract BibTeX RIS
Solid oxide fuel cells (SOFCs) are electrochemical devices often containing a ceramic electrolyte that enables the conduction of negative oxygen ions, and typically operate at elevated temperatures (i.e.,  ). Their ability to combine the exhaust hydrocarbons and heat from combustion engines to create electricity has made them a promising technology for improved fuel efficiency, as highlighted recently by Zhan and Barnett.1 At present the drive is to maintain high performance at intermediate operating temperatures (i.e.,
). Their ability to combine the exhaust hydrocarbons and heat from combustion engines to create electricity has made them a promising technology for improved fuel efficiency, as highlighted recently by Zhan and Barnett.1 At present the drive is to maintain high performance at intermediate operating temperatures (i.e.,  ), which are more suitable for small-scale applications.2 As the temperature drops, reaction kinetics at conventional cathodes, such as strontium-doped lanthanum manganite (LSMO), becomes increasingly torpid. This has led to various attempts to improve performance. One of the most straightforward, but poorly understood, areas of research is the "burn-in" phenomenon or activation, where an applied bias, or current, improves the performance of the cathode over time.3 A pronounced effect, of about an order of magnitude in decreased polarization resistance, can be seen with a very small applied current.4 For example, it was reported that the overpotential loss on a
), which are more suitable for small-scale applications.2 As the temperature drops, reaction kinetics at conventional cathodes, such as strontium-doped lanthanum manganite (LSMO), becomes increasingly torpid. This has led to various attempts to improve performance. One of the most straightforward, but poorly understood, areas of research is the "burn-in" phenomenon or activation, where an applied bias, or current, improves the performance of the cathode over time.3 A pronounced effect, of about an order of magnitude in decreased polarization resistance, can be seen with a very small applied current.4 For example, it was reported that the overpotential loss on a  electrode was reduced from initially
electrode was reduced from initially  after a cathodic current treatment at
after a cathodic current treatment at  for
for  at
at  .5 Interestingly, this phenomenon occurs for both porous screen printed cathodes6 and dense thin films.7 Unlike for Pt cathodes, where the burn-in activation processes is due to the decomposition of
.5 Interestingly, this phenomenon occurs for both porous screen printed cathodes6 and dense thin films.7 Unlike for Pt cathodes, where the burn-in activation processes is due to the decomposition of  species on the cathode surface,8 the LSMO case is more complicated. For instance, improved oxygen diffusion due to oxygen vacancy formation at high cathodic bias cannot explain the entire process; the relaxation time of the electrode performance is typically on the scale of several hours even at temperatures of
species on the cathode surface,8 the LSMO case is more complicated. For instance, improved oxygen diffusion due to oxygen vacancy formation at high cathodic bias cannot explain the entire process; the relaxation time of the electrode performance is typically on the scale of several hours even at temperatures of  rather than minutes, as would be expected from the relatively fast anion diffusion.9 Instead, the reduction is attributed to the change in the overpotentionals of the oxygen reduction reaction (ORR) at the cathode.10 A variety of mechanisms have been proposed to explain the enhanced ORR, such as changes in composition (both at the cathode surface and at the interface with the electrolyte),10–14 Mn charge state,6, 15 and morphology.5, 16
rather than minutes, as would be expected from the relatively fast anion diffusion.9 Instead, the reduction is attributed to the change in the overpotentionals of the oxygen reduction reaction (ORR) at the cathode.10 A variety of mechanisms have been proposed to explain the enhanced ORR, such as changes in composition (both at the cathode surface and at the interface with the electrolyte),10–14 Mn charge state,6, 15 and morphology.5, 16
In this study, we have used a range of synchrotron-based soft X-ray spectroscopy techniques to study the Mn charge state, chemical composition, and electronic structure of  cathodes at various stages of operation. This type of cathode is widely used because of its high electrochemical performance, thermal and chemical stability, and compatibility with the yttria-stabilized zirconia (YSZ) electrolyte (when the A-site cations are slightly deficient).17 By combining the results of Mn
cathodes at various stages of operation. This type of cathode is widely used because of its high electrochemical performance, thermal and chemical stability, and compatibility with the yttria-stabilized zirconia (YSZ) electrolyte (when the A-site cations are slightly deficient).17 By combining the results of Mn  -edge resonant photoemission spectroscopy (RPES), O
-edge resonant photoemission spectroscopy (RPES), O  -edge (and Mn
-edge (and Mn  ) X-ray absorption spectroscopy (XAS), and O
) X-ray absorption spectroscopy (XAS), and O  -edge X-ray emission spectroscopy (XES) measurements of the electronic structure of high quality dense thin films with suitable quenching and surface preparation methods, we were able to directly compare the LSMO cathode at various stages of device operation (i.e., at
-edge X-ray emission spectroscopy (XES) measurements of the electronic structure of high quality dense thin films with suitable quenching and surface preparation methods, we were able to directly compare the LSMO cathode at various stages of device operation (i.e., at  in air, before and after the activation) with its ground state at room temperature.
in air, before and after the activation) with its ground state at room temperature.
Experimental Techniques
Both XES and XAS involve a transition between electronic states within the solid, one of which is a localized core level. For O  -edge transitions (i.e., O
-edge transitions (i.e., O  2p), the measured emission or absorption spectrum can be interpreted in terms of the density of occupied valence band or unoccupied conduction band states, respectively.18, 19 Dipole selection rules (
2p), the measured emission or absorption spectrum can be interpreted in terms of the density of occupied valence band or unoccupied conduction band states, respectively.18, 19 Dipole selection rules ( ,
,  ) govern the optical transition to or from the core level,18 and in the present case O
) govern the optical transition to or from the core level,18 and in the present case O  -edge XAS and XES measurements yield information on the O 2p partial density of states (PDOS) above and below
-edge XAS and XES measurements yield information on the O 2p partial density of states (PDOS) above and below  , respectively. (The PDOS is the electronic density of states, resolved into orbital angular momentum components.) Unlike photoemission spectroscopy, these techniques are relatively insensitive to the quality of the sample surface and atomic cleaning of the surface is not necessary.
, respectively. (The PDOS is the electronic density of states, resolved into orbital angular momentum components.) Unlike photoemission spectroscopy, these techniques are relatively insensitive to the quality of the sample surface and atomic cleaning of the surface is not necessary.
Strong atomic (i.e., multiplet) effects exist in the Mn  -edge (3d-2p) XAS, due to the strong atomic overlap between the core- and valence wave functions.20 As a result, Mn
-edge (3d-2p) XAS, due to the strong atomic overlap between the core- and valence wave functions.20 As a result, Mn  -edge XAS spectra do not reflect the unoccupied Mn 3d PDOS, but can provide an accurate measure of the charge state from the multiplet line shape. RPES exploits the enhancement of the photoelectron yield for excitation energies close to an absorption threshold, such as the Mn
-edge XAS spectra do not reflect the unoccupied Mn 3d PDOS, but can provide an accurate measure of the charge state from the multiplet line shape. RPES exploits the enhancement of the photoelectron yield for excitation energies close to an absorption threshold, such as the Mn  -edge. This is due to interference between conventional photoemission and direct recombination, as reported, for example, at the transition-metal
-edge. This is due to interference between conventional photoemission and direct recombination, as reported, for example, at the transition-metal  -edge of
-edge of  and
and  .21 Excitation of an electron at the Mn
.21 Excitation of an electron at the Mn  -edge by a photon is described by
-edge by a photon is described by
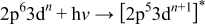
The excited state can decay by a number of mechanisms such as autoionization or direct recombination, the latter process being described by
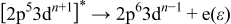
where an electron with kinetic energy  is ejected. This final state is then the same as the conventional photoemission process involving emitted 3d electrons (at this energy)
is ejected. This final state is then the same as the conventional photoemission process involving emitted 3d electrons (at this energy)
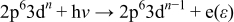
Due to the emitted electrons having the same kinetic energy, valence band photoemission spectra will display enhanced contributions from the Mn 3d electrons when the emission is excited with photon energies near the energy of the Mn  -edge. Note that RPES recorded at the Mn
-edge. Note that RPES recorded at the Mn  -edge is more useful than RPES recorded near the Mn
-edge is more useful than RPES recorded near the Mn  -edge, because there is a larger enhancement and a comparatively larger sampling depth
-edge, because there is a larger enhancement and a comparatively larger sampling depth  compared to valence band photoemission spectra excited with UV energy photons, due to the higher kinetic energy of the emitted valence band electrons.22 For example, an enhancement of the occupied Mn 3d state greater than 20 times was noted for Mn
compared to valence band photoemission spectra excited with UV energy photons, due to the higher kinetic energy of the emitted valence band electrons.22 For example, an enhancement of the occupied Mn 3d state greater than 20 times was noted for Mn  -edge RPES of
-edge RPES of  and
and  .23
.23
Experimental Details
Thin films of A-site deficient 20% strontium-doped lanthanum manganite with a chemical composition of  , were grown by pulsed laser deposition on single-crystal substrates of YSZ(111) and
, were grown by pulsed laser deposition on single-crystal substrates of YSZ(111) and  . The films were grown at the Environmental Molecular Sciences Laboratory at the Pacific Northwest National Laboratory. Films with a range of thicknesses from
. The films were grown at the Environmental Molecular Sciences Laboratory at the Pacific Northwest National Laboratory. Films with a range of thicknesses from  were grown. A Kr–F laser was used in the deposition at a laser repetition rate of
were grown. A Kr–F laser was used in the deposition at a laser repetition rate of  with a power level of
with a power level of  /pulse yielding
/pulse yielding  incident on the target. The substrates were heated to
incident on the target. The substrates were heated to  and the chamber pressure was held at an
and the chamber pressure was held at an  pressure of
pressure of  . The resulting growth rate was determined from X-ray reflectivity as
. The resulting growth rate was determined from X-ray reflectivity as  , and Rutherford backscattering spectroscopy revealed that the film composition was the same as that of the target.
, and Rutherford backscattering spectroscopy revealed that the film composition was the same as that of the target.
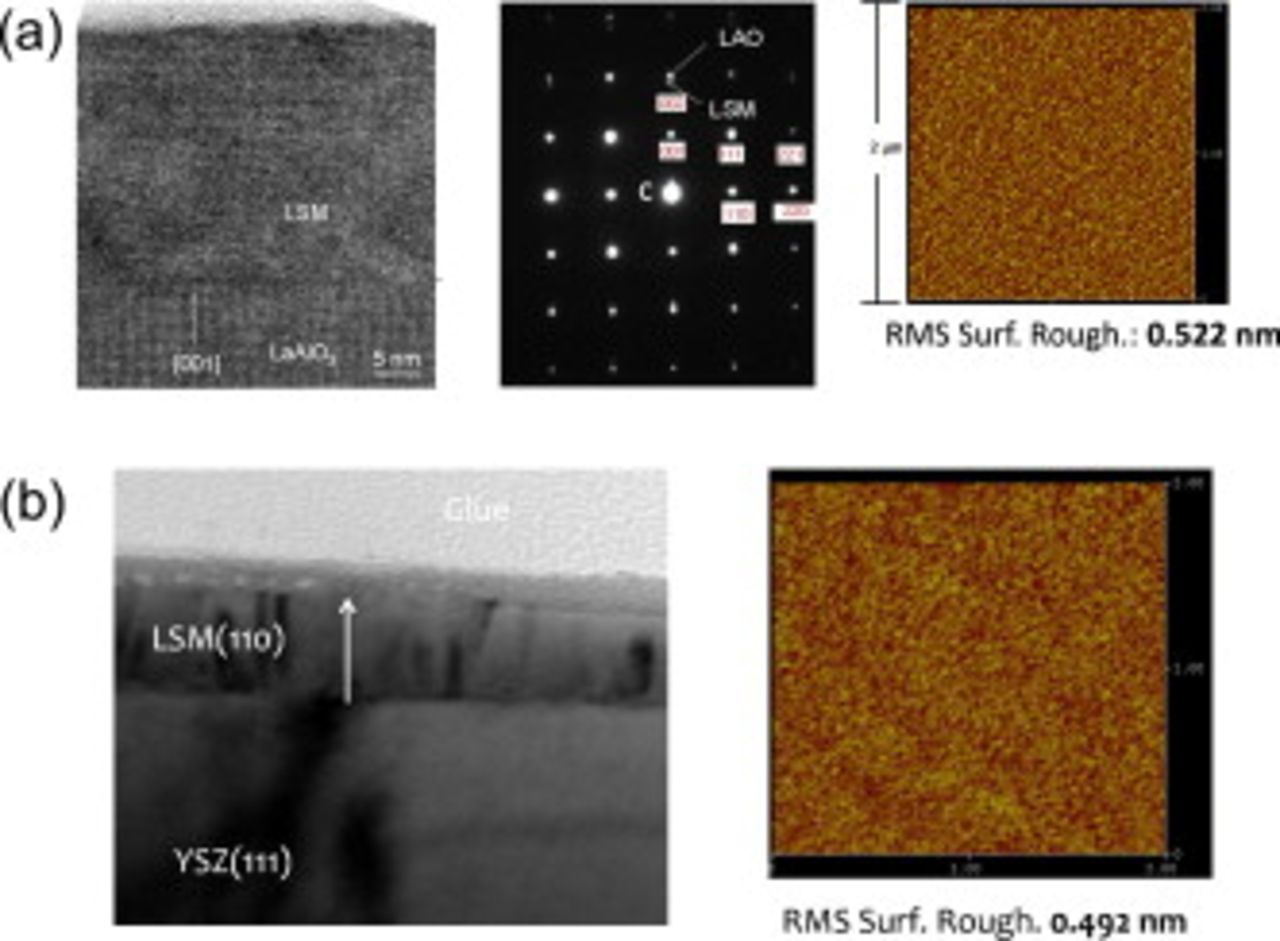
Figure 1 presents high resolution transmission electron microscopy (HRTEM) and atomic force microscopy (AFM) images used to evaluate the quality of heteroepitaxy and surface roughness of the films. LSMO films grown upon  were epitaxial, as confirmed by the HRTEM in Fig. 1a. The LSMO thin films on YSZ(111) had columnar grains oriented in the [110] growth direction but were randomly oriented in the plane, making the film surface essentially (110) as shown in the HRTEM images of Fig. 1b. AFM scans showed that the surface of the as-deposited films (independent of substrate) were very smooth, with root-mean-squared (rms) surface roughness of both samples about
were epitaxial, as confirmed by the HRTEM in Fig. 1a. The LSMO thin films on YSZ(111) had columnar grains oriented in the [110] growth direction but were randomly oriented in the plane, making the film surface essentially (110) as shown in the HRTEM images of Fig. 1b. AFM scans showed that the surface of the as-deposited films (independent of substrate) were very smooth, with root-mean-squared (rms) surface roughness of both samples about  for
for  thick samples. All experiments showed repeatable results independent of substrate and film thickness used, except for the studies where YSZ electrolyte substrates were required. HRTEM and AFM studies (not shown) of the LSMO/YSZ(111) samples revealed no increase in the surface roughness when annealed at
thick samples. All experiments showed repeatable results independent of substrate and film thickness used, except for the studies where YSZ electrolyte substrates were required. HRTEM and AFM studies (not shown) of the LSMO/YSZ(111) samples revealed no increase in the surface roughness when annealed at  for up to
for up to  . Increasing the temperature above
. Increasing the temperature above  did result in increased surface roughness compared to as-deposited films; the rms surface roughness rapidly increased to 11.6 and
did result in increased surface roughness compared to as-deposited films; the rms surface roughness rapidly increased to 11.6 and  after
after  anneals at 1100 and
anneals at 1100 and  , respectively.
, respectively.
Figure 1. The TEM and AFM results of LSMO films deposited upon (a)  and (b) YSZ(111) substrates.
and (b) YSZ(111) substrates.
Impedance spectroscopy studies were conducted in a custom chamber shown schematically in Fig. 2. A porous  thick layer of platinum was screen printed onto the opposite face from the LSM film, which was sintered at
thick layer of platinum was screen printed onto the opposite face from the LSM film, which was sintered at  for
for  . Next, current collectors consisting of a fine silver mesh followed by a coarse silver mesh with lead wires wrapped around them were attached to both sides of the sample. The sample and current collectors were loaded into the chamber, which was two mullite tubes pressing down on the sample. A thermocouple was placed within millimeters of the sample, and gas was introduced near the sample to ensure the sample was at the desired
. Next, current collectors consisting of a fine silver mesh followed by a coarse silver mesh with lead wires wrapped around them were attached to both sides of the sample. The sample and current collectors were loaded into the chamber, which was two mullite tubes pressing down on the sample. A thermocouple was placed within millimeters of the sample, and gas was introduced near the sample to ensure the sample was at the desired  . This entire setup was placed inside a closed one end mullite tube to seal off the chamber from the external atmosphere; also the mullite allowed for rapid quenching of the sample after treatment. A
. This entire setup was placed inside a closed one end mullite tube to seal off the chamber from the external atmosphere; also the mullite allowed for rapid quenching of the sample after treatment. A  bias (i.e., cathodic bias) was applied for
bias (i.e., cathodic bias) was applied for  using a potentiostat/galvanostat with a frequency response analyzer (Solartron 1280b). The same instrument was used to perform impedance measurements with a
using a potentiostat/galvanostat with a frequency response analyzer (Solartron 1280b). The same instrument was used to perform impedance measurements with a  applied ac potential at open circuit immediately after interrupting the bias treatment. After an impedance spectrum was recorded, the bias treatment was resumed. The frequency ranged from
applied ac potential at open circuit immediately after interrupting the bias treatment. After an impedance spectrum was recorded, the bias treatment was resumed. The frequency ranged from  .
.
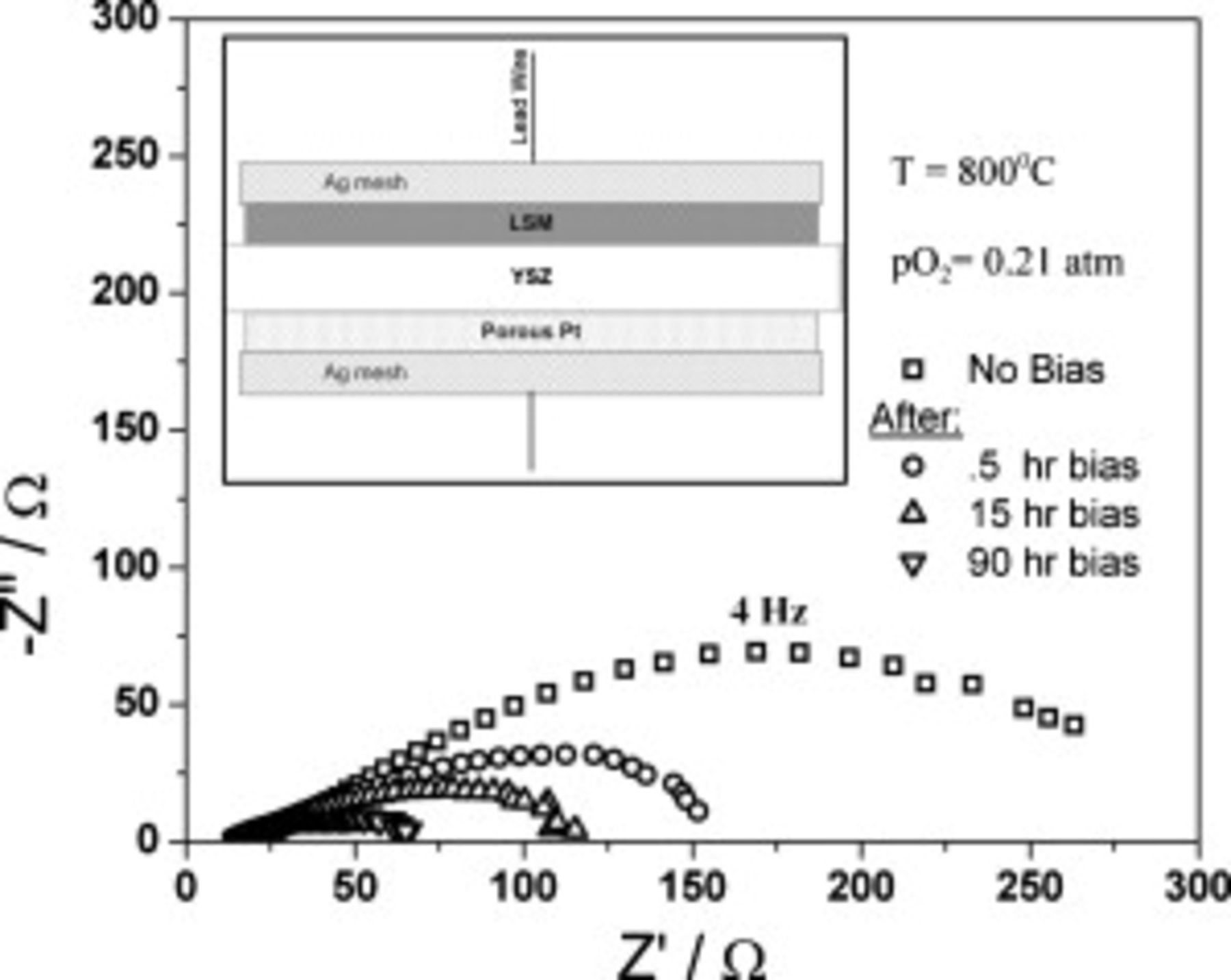
Figure 2. Nyquist plots of impedance of the LSMO/YSZ(111)/Pt SOFCs. The abscissa is a measure of real impedance while the ordinate is the imaginary part. Impedance plots taken at different times during the 
 applied bias treatment are plotted. The activation in these dense LSMO cathodes is observed by reduced impedance during continued treatment.
applied bias treatment are plotted. The activation in these dense LSMO cathodes is observed by reduced impedance during continued treatment.
Mn  -edge RPES experiments were performed at the soft X-ray undulator beamline X1B at the National Synchrotron Light Source, Brookhaven National Laboratory, which is equipped with a spherical grating monochromator. Photoemission spectra were measured in ultrahigh vacuum (UHV) using a Scienta
-edge RPES experiments were performed at the soft X-ray undulator beamline X1B at the National Synchrotron Light Source, Brookhaven National Laboratory, which is equipped with a spherical grating monochromator. Photoemission spectra were measured in ultrahigh vacuum (UHV) using a Scienta  hemispherical electron analyzer, and had a combined beamline and instrumental resolution of
hemispherical electron analyzer, and had a combined beamline and instrumental resolution of  for photon energies near the Mn
for photon energies near the Mn  -edge. Wide energy range X-ray photoemission spectroscopy (XPS) spectra of the Mn 2p and O 1s levels were measured using a photon energy of
-edge. Wide energy range X-ray photoemission spectroscopy (XPS) spectra of the Mn 2p and O 1s levels were measured using a photon energy of  , and had a combined instrumental resolution of
, and had a combined instrumental resolution of  . Binding energies are referenced relative to Au 4f photoemission from a Au foil in electrical contact with the films. Mn
. Binding energies are referenced relative to Au 4f photoemission from a Au foil in electrical contact with the films. Mn  -edge and O
-edge and O  -edge XAS spectra were measured in the surface sensitive total electron yield mode (TEY) with energy resolutions of approximately 270 and
-edge XAS spectra were measured in the surface sensitive total electron yield mode (TEY) with energy resolutions of approximately 270 and  , respectively. Beamline effects were subtracted from the TEY signal by using the background signal from a clean Au mesh in the path of the incident beam. Additional experiments were also performed on beamline 7.0.1 at the Advanced Light Source, Lawrence Berkeley National Laboratory. This beamline is also equipped with a spherical grating monochromator. Emission spectra were recorded using a Nordgren-type grazing-incidence spherical grating spectrometer.19 The O
, respectively. Beamline effects were subtracted from the TEY signal by using the background signal from a clean Au mesh in the path of the incident beam. Additional experiments were also performed on beamline 7.0.1 at the Advanced Light Source, Lawrence Berkeley National Laboratory. This beamline is also equipped with a spherical grating monochromator. Emission spectra were recorded using a Nordgren-type grazing-incidence spherical grating spectrometer.19 The O  -edge XAS was measured in both the TEY and bulk-sensitive total fluorescent yield (TFY) modes, while the Mn
-edge XAS was measured in both the TEY and bulk-sensitive total fluorescent yield (TFY) modes, while the Mn  -edge XAS was measured in the TEY mode alone. For the XAS measurements, the beamline resolution was set to similar energy resolution as at X1B and the spectra were normalized to a reference current from a clean gold mesh positioned in the path of the photon beam. O
-edge XAS was measured in the TEY mode alone. For the XAS measurements, the beamline resolution was set to similar energy resolution as at X1B and the spectra were normalized to a reference current from a clean gold mesh positioned in the path of the photon beam. O  -edge XES measurements reported here were performed at the ALS in a near-grazing geometry, with energy axes calibrated to Zn
-edge XES measurements reported here were performed at the ALS in a near-grazing geometry, with energy axes calibrated to Zn  emission lines of Zn metal in second order of diffraction.24
emission lines of Zn metal in second order of diffraction.24
Results and Discussion
Solid oxide fuel cell operation
We have performed electrochemical impedance spectroscopy (EIS) of our dense films to confirm the suitability of studying dense films for studying the activation. Figure 2 presents the impedance results from a  thick LSMO/YSZ(111) sample which was heated to
thick LSMO/YSZ(111) sample which was heated to  in air. The EIS results in Fig. 2 clearly display the activation occurring for these dense LSMO films, thus confirming their suitability for these studies. The total polarization resistance
in air. The EIS results in Fig. 2 clearly display the activation occurring for these dense LSMO films, thus confirming their suitability for these studies. The total polarization resistance  is calculated by subtracting the low frequency intercept from the high frequency intercept along the
is calculated by subtracting the low frequency intercept from the high frequency intercept along the  or real axis. It can be seen that
or real axis. It can be seen that  decreases from
decreases from  in
in  . This appears to be slower activation than was reported previously; however, the timing and extent of activation is expected to depend on a number of variables including microstructure, sample history, bias, and composition. Therefore, the reported trend and relative time scale are in agreement with other researchers. Subsequently, the impedance continued to decrease but at a slower rate. Initially, the spectra appear to contain only a single arc with a maximum frequency of
. This appears to be slower activation than was reported previously; however, the timing and extent of activation is expected to depend on a number of variables including microstructure, sample history, bias, and composition. Therefore, the reported trend and relative time scale are in agreement with other researchers. Subsequently, the impedance continued to decrease but at a slower rate. Initially, the spectra appear to contain only a single arc with a maximum frequency of  . After
. After  of applied bias, the low frequency arc has decreased to such an extent that a clear second arc emerges at a higher frequency with a peak frequency of
of applied bias, the low frequency arc has decreased to such an extent that a clear second arc emerges at a higher frequency with a peak frequency of  . We note that the Ag mesh process used to apply the cathodic bias evenly across the entire cathode rendered the surface unsuitable for photoemission experiments (including Mn
. We note that the Ag mesh process used to apply the cathodic bias evenly across the entire cathode rendered the surface unsuitable for photoemission experiments (including Mn  -edge RPES). Our measurements of the activated samples were limited to O
-edge RPES). Our measurements of the activated samples were limited to O  -edge XES/XAS and Mn
-edge XES/XAS and Mn  -edge XAS. Current efforts are underway to improve the mask design to apply an even bias and retain a suitable surface for photoemission spectroscopy.
-edge XAS. Current efforts are underway to improve the mask design to apply an even bias and retain a suitable surface for photoemission spectroscopy.
The need for UHV with current soft X-ray spectroscopy means that the majority of studies are ex situ in nature. Continued progress in spectrometer design (e.g., Ref. 25) and multistage growth/analysis beamline endstations at synchrotron facilities (e.g., MaxLab and Spring8) have facilitated a few in situ studies, where the samples have either been grown and/or exposed to bias and higher pressures and directly measured within the same system. We stress that our X-ray spectroscopy measurements are ex situ studies, but care has been taken to preserve the final condition of the as-processed, exposed, and activated cathodes. In order to retain the final condition of our films we used a rapid quenching method to study samples exposed to SOFC atmospheric conditions and operation (i.e.,  to room temperature within
to room temperature within  ) for LSMO films exposed at
) for LSMO films exposed at  in air without a bias and with a
in air without a bias and with a  bias. Our study also included LSMO films cooled in thermal equilibrium in order to determine the ability of the quenching to preserve their final state (which will be discussed further in the next section). The samples were sealed in evacuated glass vials until released and mounted within a dry
bias. Our study also included LSMO films cooled in thermal equilibrium in order to determine the ability of the quenching to preserve their final state (which will be discussed further in the next section). The samples were sealed in evacuated glass vials until released and mounted within a dry  glove box environment and immediately inserted into the UHV analysis chambers. Exposure to atmospheric conditions was limited to at most
glove box environment and immediately inserted into the UHV analysis chambers. Exposure to atmospheric conditions was limited to at most  , which will be shown using XPS to result in minimal contamination (e.g., C–OH). Any comparisons between our results and in situ X-ray spectroscopy studies reported elsewhere are clearly identified below.
, which will be shown using XPS to result in minimal contamination (e.g., C–OH). Any comparisons between our results and in situ X-ray spectroscopy studies reported elsewhere are clearly identified below.
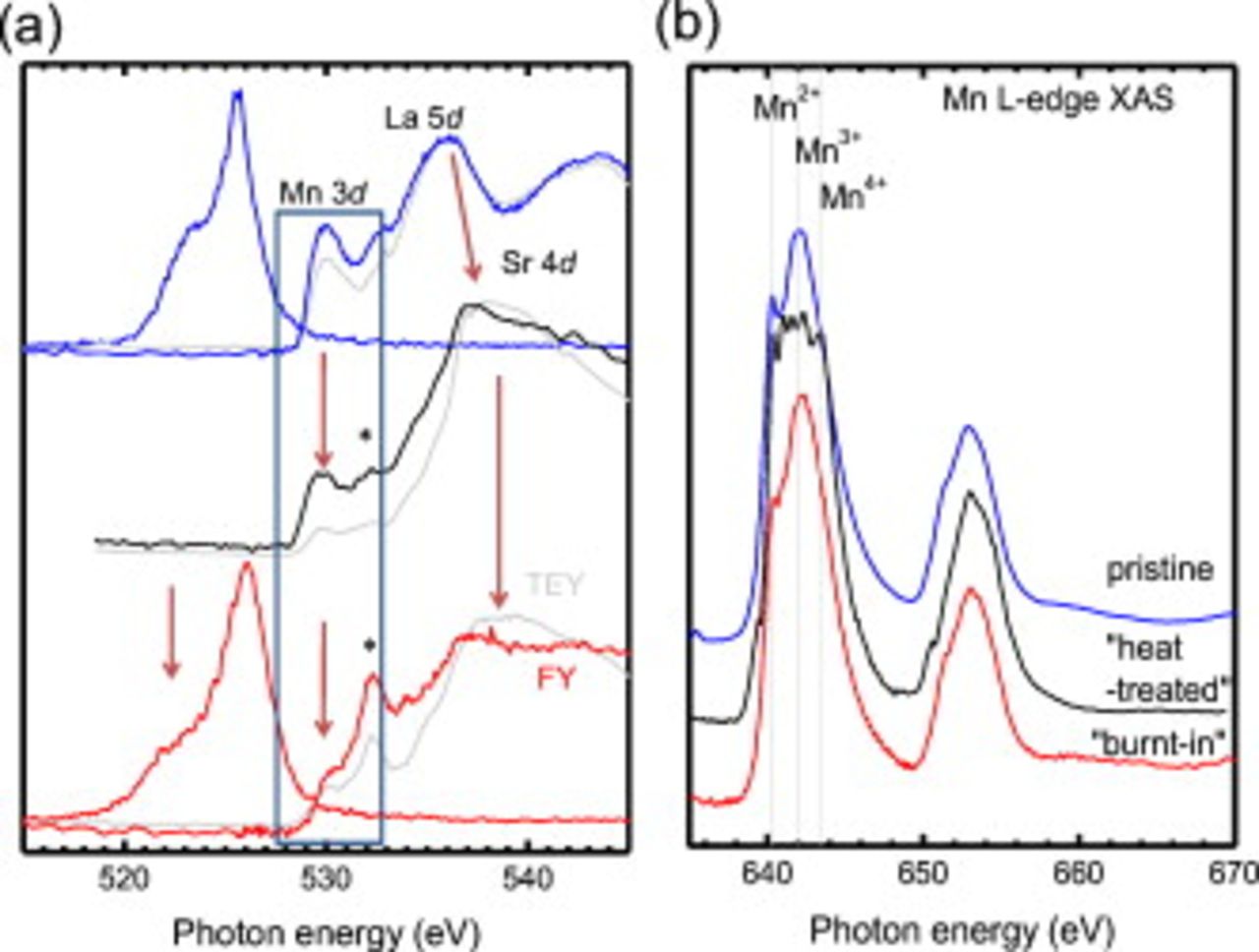
Figures 3a and 3b display a direct comparison between the O  -edge XES/XAS and Mn
-edge XES/XAS and Mn  -edge XAS of pristine and rapidly quenched activated films, respectively. For completeness we have also included the results of a reference rapidly quenched exposed
-edge XAS of pristine and rapidly quenched activated films, respectively. For completeness we have also included the results of a reference rapidly quenched exposed  thick LSMO film deposited upon a YSZ(111) substrate, which was exposed to the same atmospheric conditions as the activated sample for
thick LSMO film deposited upon a YSZ(111) substrate, which was exposed to the same atmospheric conditions as the activated sample for  with no bias and was quenched, sealed, and mounted in the same manner as the activated LSMO.
with no bias and was quenched, sealed, and mounted in the same manner as the activated LSMO.
Figure 3. (a) O  -edge XES/XAS and (b) Mn
-edge XES/XAS and (b) Mn  -edge XAS of LSMO as-processed (blue), SOFC environment exposed (black), and activated (red) films. For XAS both TEY (colored) and TFY (gray) spectra are shown. The Mn charge-state multiplet line shape peak energies are plotted as vertical dashed lines. Changes in the O
-edge XAS of LSMO as-processed (blue), SOFC environment exposed (black), and activated (red) films. For XAS both TEY (colored) and TFY (gray) spectra are shown. The Mn charge-state multiplet line shape peak energies are plotted as vertical dashed lines. Changes in the O  -edge XES/XAS are indentified by arrows. The addition of a peak
-edge XES/XAS are indentified by arrows. The addition of a peak  in the highlighted hybridized Mn 3d-O 2p prepeak region (blue box) is shown.
in the highlighted hybridized Mn 3d-O 2p prepeak region (blue box) is shown.
Referring first to the pristine case, we observe for the O  -edge XAS: (1) a pre-edge region associated with covalent mixing of the O 2p and Mn 3d unoccupied states
-edge XAS: (1) a pre-edge region associated with covalent mixing of the O 2p and Mn 3d unoccupied states  ; (2) an intense asymmetric peak due to both hybridized O 2p–La 4d and O 2p–Sr 4d unoccupied states
; (2) an intense asymmetric peak due to both hybridized O 2p–La 4d and O 2p–Sr 4d unoccupied states  ; and (3) a broad peak due to O 2p–Mn 4s,p unoccupied states.26 The two modes measure different electron–hole recombination channels. The TEY mode measures mainly the secondary electrons associated with nonradiative Auger processes following the excitation of a core-electron into the conduction band, while the TFY mode measures the X-ray photons emitted following the excitation. XAS recorded in a TEY mode has a surface sensitivity of
; and (3) a broad peak due to O 2p–Mn 4s,p unoccupied states.26 The two modes measure different electron–hole recombination channels. The TEY mode measures mainly the secondary electrons associated with nonradiative Auger processes following the excitation of a core-electron into the conduction band, while the TFY mode measures the X-ray photons emitted following the excitation. XAS recorded in a TEY mode has a surface sensitivity of  , while XAS recorded in a TFY mode has an effective probing depth of roughly
, while XAS recorded in a TFY mode has an effective probing depth of roughly  . The similarity between the TEY and TFY is consistent with a homogeneous sample, and the observed spectral shape is also in agreement with that expected for a Sr doping of approximately 20%.26 Also shown in Fig. 3a is the corresponding above-threshold O
. The similarity between the TEY and TFY is consistent with a homogeneous sample, and the observed spectral shape is also in agreement with that expected for a Sr doping of approximately 20%.26 Also shown in Fig. 3a is the corresponding above-threshold O  -edge XES spectrum
-edge XES spectrum  of the pristine sample, where the incident energy is sufficient to excite the electron completely out of the solid. The combination of dipole-selection rules, energy considerations, and the "photon-in, photon-out" nature of XES means that we measure the bulk O 2p contribution to the valence band region, in contrast to traditional XPS, which measures the total density of states. We observe the same asymmetric O
of the pristine sample, where the incident energy is sufficient to excite the electron completely out of the solid. The combination of dipole-selection rules, energy considerations, and the "photon-in, photon-out" nature of XES means that we measure the bulk O 2p contribution to the valence band region, in contrast to traditional XPS, which measures the total density of states. We observe the same asymmetric O  -edge spectral shape as for other hole-doped manganite systems, including more importantly the parent material
-edge spectral shape as for other hole-doped manganite systems, including more importantly the parent material  .27, 28 We are also able to clearly resolve the lower photon energy (higher binding) energy shoulder at
.27, 28 We are also able to clearly resolve the lower photon energy (higher binding) energy shoulder at  in addition to the main peak at
in addition to the main peak at  , in contrast to recent O
, in contrast to recent O  -edge XES of
-edge XES of  reported by Manella et al.29 Meanwhile, the corresponding Mn
reported by Manella et al.29 Meanwhile, the corresponding Mn  -edge XAS spectrum in Fig. 3b displays two broad multiplet features, separated by the energy of the spin-orbit split Mn 2p core level. To a first approximation, the Mn
-edge XAS spectrum in Fig. 3b displays two broad multiplet features, separated by the energy of the spin-orbit split Mn 2p core level. To a first approximation, the Mn  -edge can be understood using ligand-field multiplet theory for
-edge can be understood using ligand-field multiplet theory for  model calculations within
model calculations within  symmetry.26 It has been experimentally shown that the valence of the Mn ions of hole-doped
symmetry.26 It has been experimentally shown that the valence of the Mn ions of hole-doped  systems can be considered as a linear combination of the
systems can be considered as a linear combination of the  and
and  end members (i.e.,
end members (i.e.,  ),30 hence the observed similarity with the
),30 hence the observed similarity with the  end member for this hole doping (i.e.,
end member for this hole doping (i.e.,  ). The greatest difference between our pristine case and the parent material is the shift of the
). The greatest difference between our pristine case and the parent material is the shift of the  multiple peak to
multiple peak to  (compared to
(compared to  for
for  ), which is consistent with the earlier Sr-doping studies of Abbate et al.26 For completeness we have included the photon energy of the peak intensity of the multiplet line shape associated with Mn
), which is consistent with the earlier Sr-doping studies of Abbate et al.26 For completeness we have included the photon energy of the peak intensity of the multiplet line shape associated with Mn  -edge XAS of
-edge XAS of  ,
,  , and
, and  by Gilbert et al.31 Overall, our results would indicate that the as-processed LSMO films are uniform and consistent with
by Gilbert et al.31 Overall, our results would indicate that the as-processed LSMO films are uniform and consistent with  from comparison with Mn
from comparison with Mn  -edge XAS studies of hole-doped
-edge XAS studies of hole-doped  .26, 28
.26, 28
We next refer to the activated case, where the O  -edge region is severely altered while the Mn
-edge region is severely altered while the Mn  -edge appears relatively unchanged in Fig. 3. In the O
-edge appears relatively unchanged in Fig. 3. In the O  -edge XES there is a reduction of spectral intensity (indicated by arrow) at
-edge XES there is a reduction of spectral intensity (indicated by arrow) at  following the activation. From our analysis of the pristine case, this would correspond to a reduction of Mn 3d
following the activation. From our analysis of the pristine case, this would correspond to a reduction of Mn 3d  and
and  bands hybridization with the apical O
bands hybridization with the apical O  orbitals. This is further supported by the reduction of the Mn 3d–O 2p hybridized prepeak region at
orbitals. This is further supported by the reduction of the Mn 3d–O 2p hybridized prepeak region at  in the accompanying O
in the accompanying O  -edge XAS, which would suggest a severe change in the Mn–O environment. We also note the energetic shift of the spectral weight at
-edge XAS, which would suggest a severe change in the Mn–O environment. We also note the energetic shift of the spectral weight at  (due to La 5d–O 2p hybridization) toward
(due to La 5d–O 2p hybridization) toward  . This shift would be expected for increased Sr content nearer the surface (i.e., Sr enrichment), due to increased Sr 4d–O 2p hybridization, as reported by Abbate et al.26 However, the spectral shape is not consistent with a uniform increase in hole doping alone, where the pre-edge region
. This shift would be expected for increased Sr content nearer the surface (i.e., Sr enrichment), due to increased Sr 4d–O 2p hybridization, as reported by Abbate et al.26 However, the spectral shape is not consistent with a uniform increase in hole doping alone, where the pre-edge region  should increase in intensity. The emergence of the peak at
should increase in intensity. The emergence of the peak at  (indicated by
(indicated by  ) in Fig. 3a would also suggest the formation of new Mn-oxide species,31 and/or SrO formation.32 The corresponding reduction of the
) in Fig. 3a would also suggest the formation of new Mn-oxide species,31 and/or SrO formation.32 The corresponding reduction of the  contribution to the Mn
contribution to the Mn  -edge XAS in Fig. 3b following application of the bias would immediately rule out MnO formation expected, at least within the near-surface region. This result would appear consistent with the migration of
-edge XAS in Fig. 3b following application of the bias would immediately rule out MnO formation expected, at least within the near-surface region. This result would appear consistent with the migration of  species toward the LSMO/electrolyte interface (i.e., reduced
species toward the LSMO/electrolyte interface (i.e., reduced  species near the LSMO/air interface) to promote direct incorporation of oxygen into the electrolyte proposed by in situ photoemission microscopy (
species near the LSMO/air interface) to promote direct incorporation of oxygen into the electrolyte proposed by in situ photoemission microscopy ( , anodic and cathodic polarization of
, anodic and cathodic polarization of  ) studies of Backhaus-Ricoult et al.6 The observed
) studies of Backhaus-Ricoult et al.6 The observed  charge state would suggest the formation of
charge state would suggest the formation of  ,31 or even
,31 or even  proposed by la O' et al.7 The latter was considered by la O' et al. to be partly responsible for the enhanced ORR after application of a cathodic potential with their quenched samples.
proposed by la O' et al.7 The latter was considered by la O' et al. to be partly responsible for the enhanced ORR after application of a cathodic potential with their quenched samples.
The lack of change in the Mn charge state upon application of the cathodic bias would immediately appear to conflict with various photoemission studies.6, 33 Comparison with the exposed sample in Fig. 3 provides further insight. The evolution of the O  -edge in Fig. 3a suggests that the possible formation of the
-edge in Fig. 3a suggests that the possible formation of the  and/or passive
and/or passive  species appears to occur upon heating alone, in contrast to the bias-driven observations by la O' et al.7 Certainly, the oxygen local environment is altered most significantly at elevated temperatures rather than upon application of a bias. Minor alterations in the relative spectral intensity in the pre-edge region (i.e.,
species appears to occur upon heating alone, in contrast to the bias-driven observations by la O' et al.7 Certainly, the oxygen local environment is altered most significantly at elevated temperatures rather than upon application of a bias. Minor alterations in the relative spectral intensity in the pre-edge region (i.e.,  ) of the O
) of the O  -edge XAS are noted between "heat-treated" and "burnt-in" quenched LSMO. In contrast to the pristine case, there is also increased depth variation (as noted between the TEY and TFY modes). This appears most apparent in the heat-treated case, although it is still present for the burnt-in case. From comparison with the heat-treated Mn
-edge XAS are noted between "heat-treated" and "burnt-in" quenched LSMO. In contrast to the pristine case, there is also increased depth variation (as noted between the TEY and TFY modes). This appears most apparent in the heat-treated case, although it is still present for the burnt-in case. From comparison with the heat-treated Mn  -edge XA spectrum in Fig. 3b, this difference in the XAS results recorded in TFY and TEY at the O
-edge XA spectrum in Fig. 3b, this difference in the XAS results recorded in TFY and TEY at the O  -edge could be due to the increased MnO
-edge could be due to the increased MnO  contribution nearer the surface. As noted previously, the
contribution nearer the surface. As noted previously, the  ions could migrate toward the interface upon application of a bias as suggested by in situ studies of Backhaus-Ricoult et al.6 and thus explain their absence in the Mn
ions could migrate toward the interface upon application of a bias as suggested by in situ studies of Backhaus-Ricoult et al.6 and thus explain their absence in the Mn  -edge XAS results from the burnt-in sample. Finally, we observe the shift in spectral weight in the Mn
-edge XAS results from the burnt-in sample. Finally, we observe the shift in spectral weight in the Mn  -edge XAS for the heat-treated case that is consistent with increased
-edge XAS for the heat-treated case that is consistent with increased  contributions. Our results do display the expected reduction of the Mn species following cathodic bias as reported in photoemission studies,6, 33 but before the application of the bias there is an increased
contributions. Our results do display the expected reduction of the Mn species following cathodic bias as reported in photoemission studies,6, 33 but before the application of the bias there is an increased  contribution (at least nearest the surface) likely due to Sr enrichment upon heating.
contribution (at least nearest the surface) likely due to Sr enrichment upon heating.
Initial state of operation
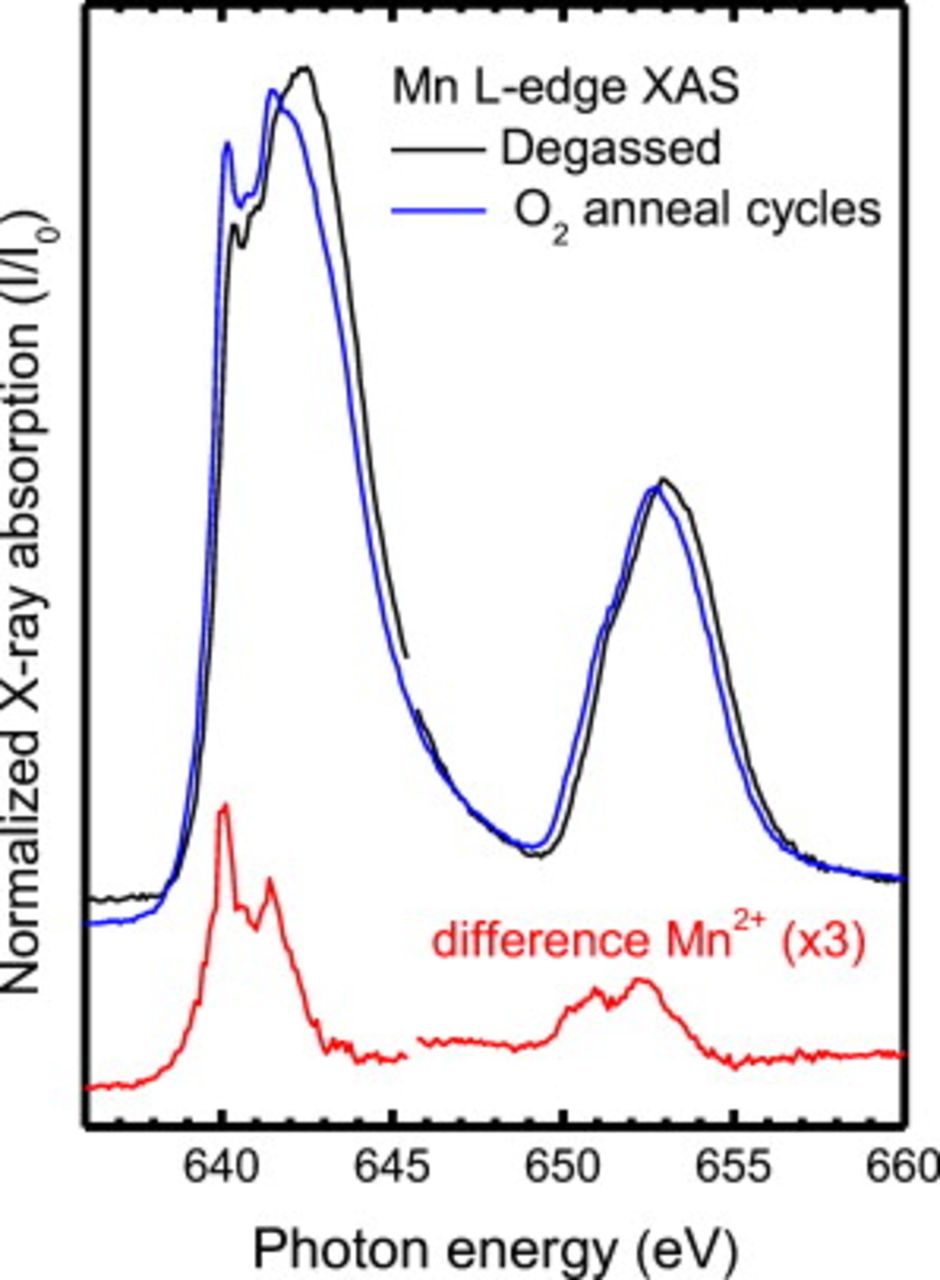
The remainder of the work presented here focuses on further understanding the chemical and electronic modification of the LSMO cathode at elevated temperatures and in air at atmospheric pressures before the application of a bias. These samples can be studied with XPS (in contrast to the burnt-in samples). The use of photoemission typically requires some cleaning of the sample surface, which can result in the modification of the surface electronic and chemical structure due to annealing and ion sputtering cycles. The presence of  (associated with Mn reduction at elevated temperatures) can complicate the Mn
(associated with Mn reduction at elevated temperatures) can complicate the Mn  -edge RPES of LSMO, as highlighted recently by de Jong et al.34 Figure 4 displays the Mn
-edge RPES of LSMO, as highlighted recently by de Jong et al.34 Figure 4 displays the Mn  -edge XAS of pristine
-edge XAS of pristine  thick
thick  following a low temperature
following a low temperature  anneal for
anneal for  in UHV, and after a cleaning cycle involving a
in UHV, and after a cleaning cycle involving a 
 ion bombardment (ion
ion bombardment (ion  ; pressure of Ar in the sample
; pressure of Ar in the sample  ) followed by two cycles of annealing in an
) followed by two cycles of annealing in an  partial pressure for
partial pressure for  (
(
 ; annealing temperature
; annealing temperature  ). The corresponding wide energy range XPS (not shown) following the cleaning cycle revealed no carbon signal along with a single asymmetric O 1s peak (binding
). The corresponding wide energy range XPS (not shown) following the cleaning cycle revealed no carbon signal along with a single asymmetric O 1s peak (binding  ), consistent with
), consistent with  reported from in situ grown LSMO.35 The Mn
reported from in situ grown LSMO.35 The Mn  -edge XAS (before the cleaning cycles) is in agreement with previous reports of
-edge XAS (before the cleaning cycles) is in agreement with previous reports of  with similar compositions.26, 34 Furthermore, we note agreement with similar studies of
with similar compositions.26, 34 Furthermore, we note agreement with similar studies of  and hole-doped
and hole-doped  (for
(for  ,
,  earth metal).26–28 After surface cleaning, a strong pre-edge peak emerges in the XAS spectrum at
earth metal).26–28 After surface cleaning, a strong pre-edge peak emerges in the XAS spectrum at  , accompanied with a shift of the main
, accompanied with a shift of the main  peak
peak  toward lower photon energies. The difference spectrum between the two spectra resembles a typical
toward lower photon energies. The difference spectrum between the two spectra resembles a typical  spectrum and highlights the strong susceptibility of these films to
spectrum and highlights the strong susceptibility of these films to  formation at the surfaces even in
formation at the surfaces even in  partial pressures (up to
partial pressures (up to  ). Continued cleaning cycles resulted in a stronger
). Continued cleaning cycles resulted in a stronger  multiplet signature in the Mn
multiplet signature in the Mn  -edge XAS and the corresponding O
-edge XAS and the corresponding O  -edge XAS (not shown here) was in agreement with MnO XAS reported elsewhere.31 All photoemission spectra reported in this study are from LSMO, which displayed no increased
-edge XAS (not shown here) was in agreement with MnO XAS reported elsewhere.31 All photoemission spectra reported in this study are from LSMO, which displayed no increased  multiplet signature in the corresponding XAS difference spectra.
multiplet signature in the corresponding XAS difference spectra.
Figure 4. Mn  -edge XAS spectra of degassed and
-edge XAS spectra of degassed and  annealed
annealed  films. The difference spectrum is plotted underneath (red).
films. The difference spectrum is plotted underneath (red).
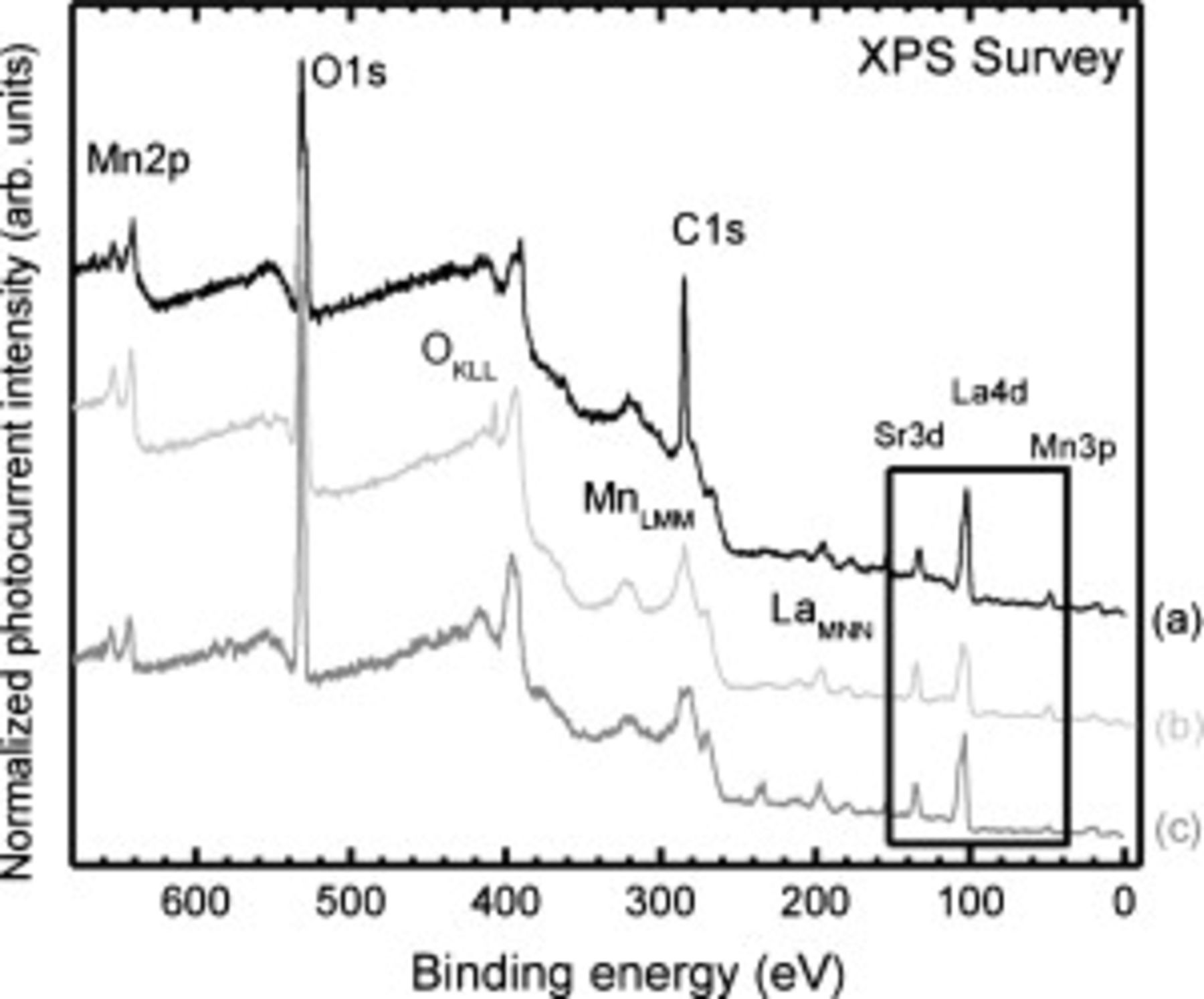
Figure 5 displays wide energy range XPS survey scans of the pristine  thick LSMO/YSZ(111) and the heat-treated
thick LSMO/YSZ(111) and the heat-treated  thick
thick  films (both equilibrium cooled and rapidly quenched). There is a lack of significant C 1s signal (due to surface C–H) on heat-treated samples, which is in agreement with the reported cleanliness of LSMO cathodes studied using in situ photoemission microscopy by Backhaus-Ricoult et al.6 It also demonstrates our ability to quench, seal, and transfer treated samples with minimal atmospheric exposure. Our measurements clearly show that upon heat-treatment the quenched film is La-deficient at least close to the surface (i.e., increased
films (both equilibrium cooled and rapidly quenched). There is a lack of significant C 1s signal (due to surface C–H) on heat-treated samples, which is in agreement with the reported cleanliness of LSMO cathodes studied using in situ photoemission microscopy by Backhaus-Ricoult et al.6 It also demonstrates our ability to quench, seal, and transfer treated samples with minimal atmospheric exposure. Our measurements clearly show that upon heat-treatment the quenched film is La-deficient at least close to the surface (i.e., increased  ratio compared to the pristine case), whereas cooling in equilibrium returns the chemical compositions tending to the pristine case, as expected for stable films. These results support earlier secondary-ion mass spectrometry depth profiles of annealed LSMO pellets by Fearn et al. ,36 which revealed excess Sr and depleted La at the surface compared to the bulk at elevated temperatures in air. However, these measurements do not rule out the possibility of phase separation that may still exist. This is further considered from examination of our corresponding XAS measurements below.
ratio compared to the pristine case), whereas cooling in equilibrium returns the chemical compositions tending to the pristine case, as expected for stable films. These results support earlier secondary-ion mass spectrometry depth profiles of annealed LSMO pellets by Fearn et al. ,36 which revealed excess Sr and depleted La at the surface compared to the bulk at elevated temperatures in air. However, these measurements do not rule out the possibility of phase separation that may still exist. This is further considered from examination of our corresponding XAS measurements below.
Figure 5. Wide-scan XPS surveys of (a) as-processed films and those heated to  in air and then (b) rapidly quenched or (c) cooled in thermal equilibrium. The La 4d, Sr 3d, and Mn 3p region is highlighted.
in air and then (b) rapidly quenched or (c) cooled in thermal equilibrium. The La 4d, Sr 3d, and Mn 3p region is highlighted.
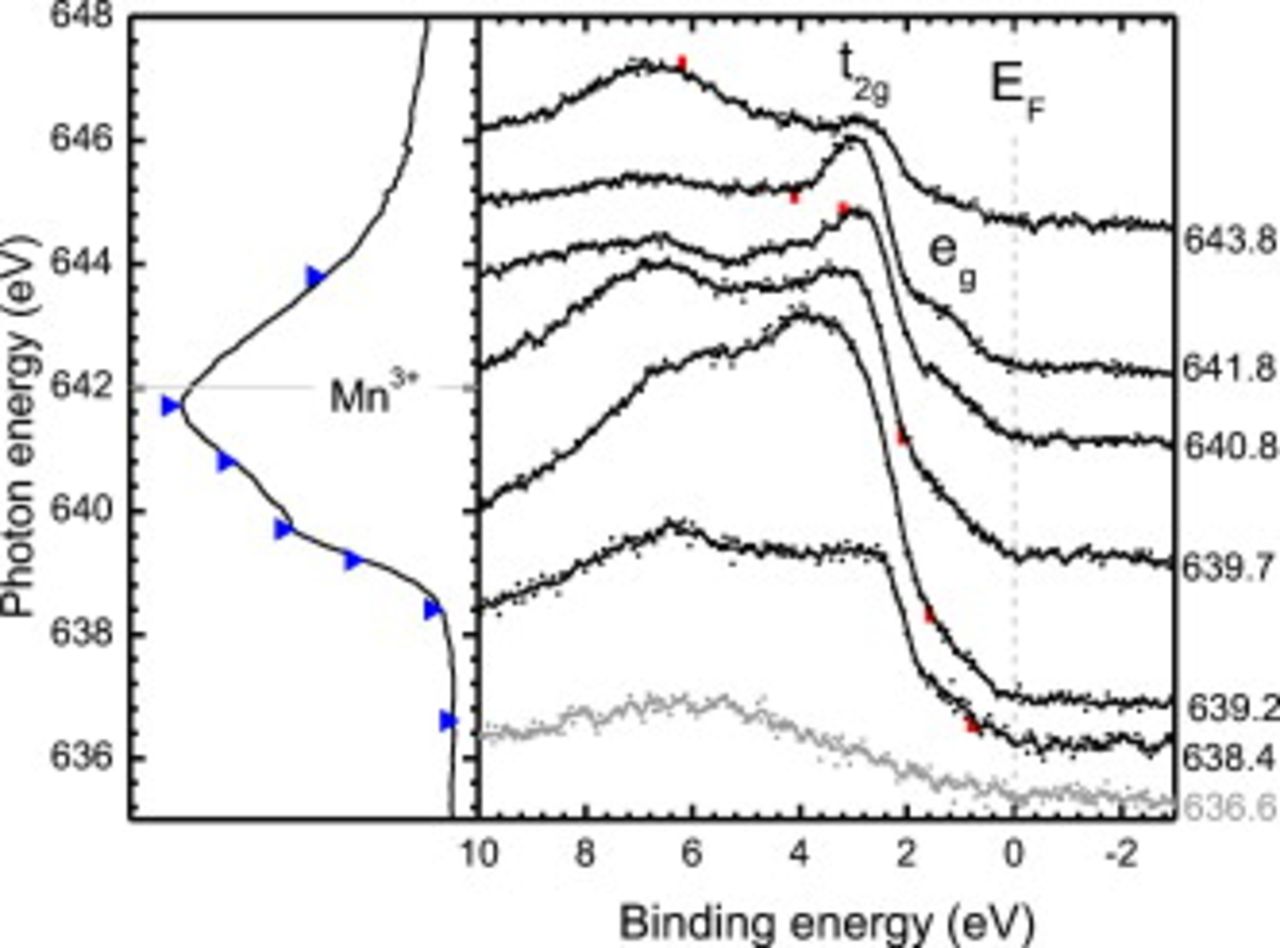
Figure 6 displays the Mn  -edge RPES spectra from the equilibrium-cooled
-edge RPES spectra from the equilibrium-cooled  thick
thick  films. The intensities of the photoemission spectra have been normalized by the secondary electron background at a binding energy of
films. The intensities of the photoemission spectra have been normalized by the secondary electron background at a binding energy of  (i.e., away from any spectral feature), and clearly illustrate the enhanced Mn 3d contribution (i.e., peaks at
(i.e., away from any spectral feature), and clearly illustrate the enhanced Mn 3d contribution (i.e., peaks at  and
and  ) at resonance for this sample. For incident X-ray excitations below the Mn
) at resonance for this sample. For incident X-ray excitations below the Mn  -edge onset, the valence band region lacks significant spectral weight below
-edge onset, the valence band region lacks significant spectral weight below  . The similar order of magnitude of the Mn 3d and O 2p cross sections at these photon energies37 means that the absence of the Mn 3d states near the Fermi level is associated mostly with the surface disorder in the degassed sample, as proposed by Horiba et al. from their in situ XPS measurement of LSMO films.35 The valence band region off-resonance is in agreement with similar photoemission studies of
. The similar order of magnitude of the Mn 3d and O 2p cross sections at these photon energies37 means that the absence of the Mn 3d states near the Fermi level is associated mostly with the surface disorder in the degassed sample, as proposed by Horiba et al. from their in situ XPS measurement of LSMO films.35 The valence band region off-resonance is in agreement with similar photoemission studies of  surfaces by de Jong et al. ,34 and its spectral shape largely stems from the O 2p contribution.38 A combined O
surfaces by de Jong et al. ,34 and its spectral shape largely stems from the O 2p contribution.38 A combined O  -edge XES, Mn
-edge XES, Mn  -edge RPES and RIXS study of pristine LSMO
-edge RPES and RIXS study of pristine LSMO  confirmed the assignment of these orbitals. We also note the strong similarity between the Mn
confirmed the assignment of these orbitals. We also note the strong similarity between the Mn  -edge RPES of our equilibrium cooled LSMO and the virgin LSMO from the same growth. For the virgin LSMO, the partial filling of the LSMO spectral weight at the Fermi level is evidence of the metallic nature of these films for this composition (i.e.,
-edge RPES of our equilibrium cooled LSMO and the virgin LSMO from the same growth. For the virgin LSMO, the partial filling of the LSMO spectral weight at the Fermi level is evidence of the metallic nature of these films for this composition (i.e.,  ) at
) at  .39 This was further supported by a measured resistivity of
.39 This was further supported by a measured resistivity of  at room temperature.
at room temperature.
Figure 6. (Left) Mn  -edge XAS of equilibrium-cooled heat-treated LSMO with the incident photon energies indicated (blue triangles). The
-edge XAS of equilibrium-cooled heat-treated LSMO with the incident photon energies indicated (blue triangles). The  charge-state multiplet line shape peak energy is plotted as horizontal dashed lines. (Right) The corresponding normalized Mn
charge-state multiplet line shape peak energy is plotted as horizontal dashed lines. (Right) The corresponding normalized Mn  -edge RPES and nonresonant photoemission spectrum of the valence band region are plotted, with respect to binding energy referenced to the Fermi edge of Au. The contribution from the
-edge RPES and nonresonant photoemission spectrum of the valence band region are plotted, with respect to binding energy referenced to the Fermi edge of Au. The contribution from the  Auger emission line is identified for each spectrum by the red vertical lines.
Auger emission line is identified for each spectrum by the red vertical lines.
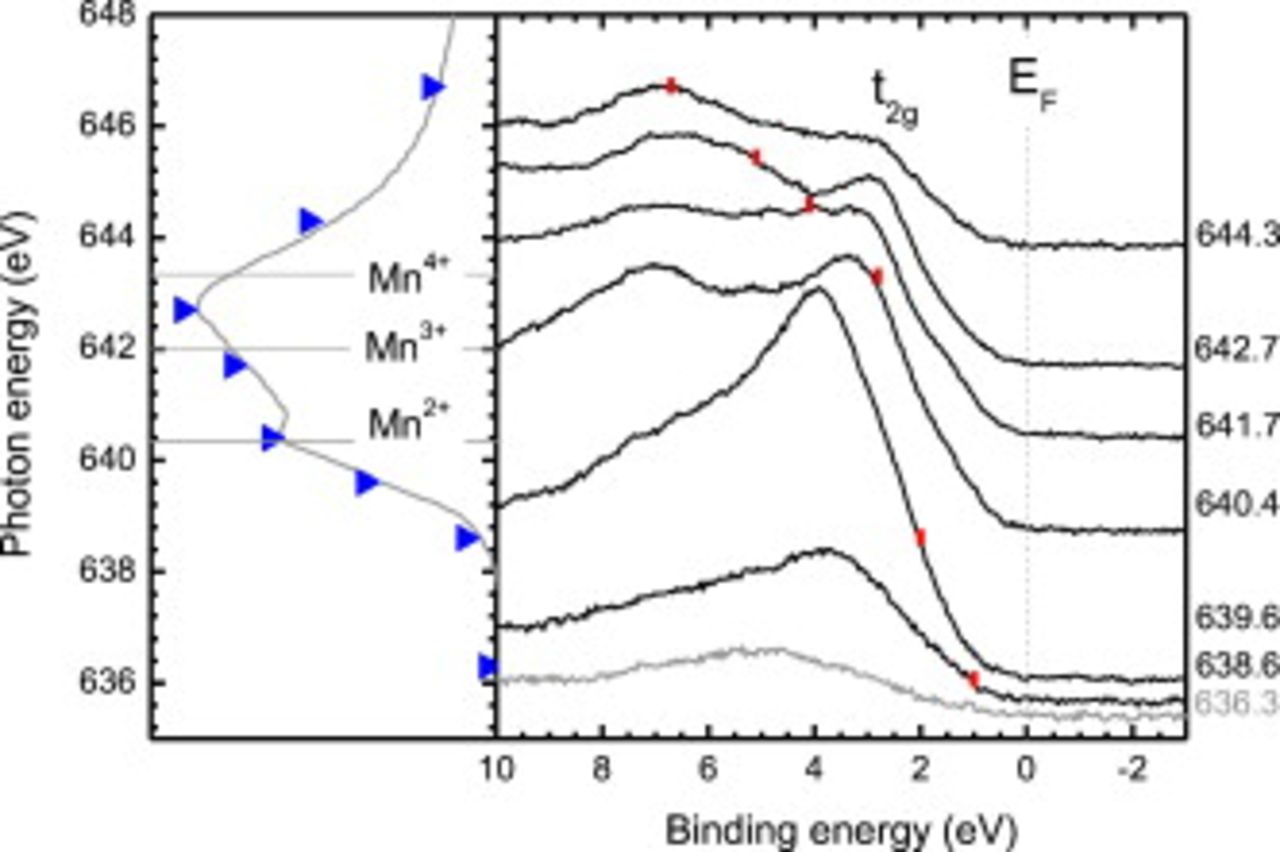
Figure 7 displays the corresponding Mn  -edge RPES of the quenched LSMO film. From comparison with Fig. 6, the greatest difference is the absence of the
-edge RPES of the quenched LSMO film. From comparison with Fig. 6, the greatest difference is the absence of the  -state and spectral weight at the Fermi level indicating an insulating character. In view of the evidence regarding reduced
-state and spectral weight at the Fermi level indicating an insulating character. In view of the evidence regarding reduced  ratio from the wide-scan XPS (Fig. 5), we consider the Mn
ratio from the wide-scan XPS (Fig. 5), we consider the Mn  -edge RPES to be consistent with the expected increased hole doping compared to the reference LSMO (i.e.,
-edge RPES to be consistent with the expected increased hole doping compared to the reference LSMO (i.e.,  ) associated with the reduced
) associated with the reduced  ratio. The absence of the
ratio. The absence of the  state
state  and the insulating character of the heat-treated and quenched LSMO would suggest hole doping of
and the insulating character of the heat-treated and quenched LSMO would suggest hole doping of  .40 Finally, the similarity of the
.40 Finally, the similarity of the  energetic region between the pristine and quenched LSMO films is unsurprising considering the similarity of the valence-band XPS as a function of Sr content reported elsewhere, e.g., Ref. 35, 38.
energetic region between the pristine and quenched LSMO films is unsurprising considering the similarity of the valence-band XPS as a function of Sr content reported elsewhere, e.g., Ref. 35, 38.
Figure 7. (Left) Mn  -edge XAS of rapidly quenched heat-treated LSMO with the incident photon energies indicated (blue triangles). The Mn charge-state multiplet line shape peak energies are plotted as horizontal dashed lines. (Right) The corresponding normalized Mn
-edge XAS of rapidly quenched heat-treated LSMO with the incident photon energies indicated (blue triangles). The Mn charge-state multiplet line shape peak energies are plotted as horizontal dashed lines. (Right) The corresponding normalized Mn  -edge RPES and nonresonant photoemission spectrum of the valence band region are plotted, with respect to binding energy referenced to the Fermi edge of Au. The contribution from the
-edge RPES and nonresonant photoemission spectrum of the valence band region are plotted, with respect to binding energy referenced to the Fermi edge of Au. The contribution from the  Auger emission line in each photoemission spectrum is identified by the red vertical lines.
Auger emission line in each photoemission spectrum is identified by the red vertical lines.
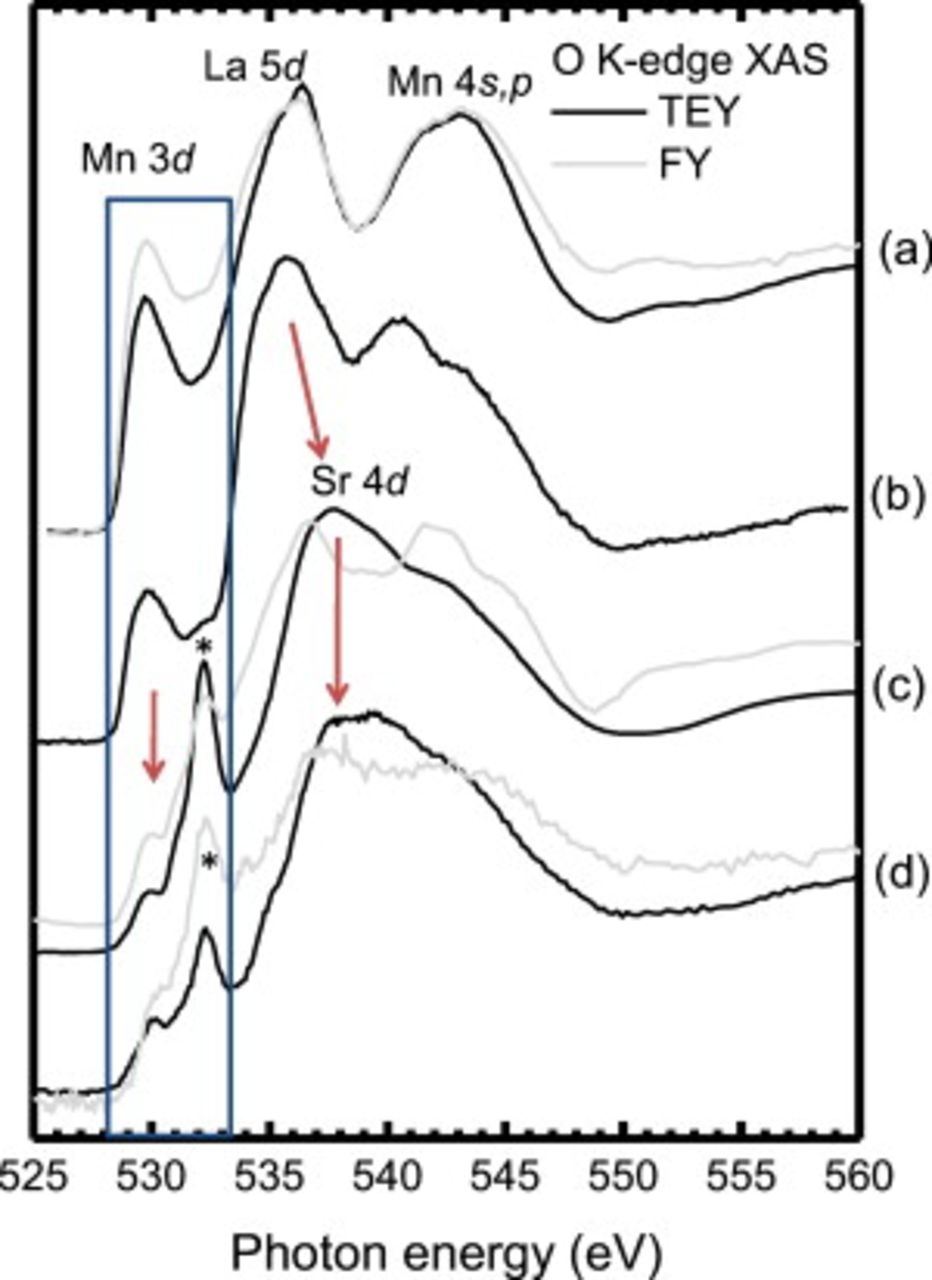
The corresponding O  -edge XAS spectra are presented in Fig. 8. Upon heat-treatment the equilibrium cooled and quenched samples behave differently. The equilibrium cooled recovered the pristine LSMO spectral shape, whereas the quenched case, reflecting the sample at SOFC atmospheric conditions, displayed dramatic changes (i.e., the reduction of the peak at
-edge XAS spectra are presented in Fig. 8. Upon heat-treatment the equilibrium cooled and quenched samples behave differently. The equilibrium cooled recovered the pristine LSMO spectral shape, whereas the quenched case, reflecting the sample at SOFC atmospheric conditions, displayed dramatic changes (i.e., the reduction of the peak at  , the emergence of a peak at
, the emergence of a peak at  , and the formation of an intense broad peak
, and the formation of an intense broad peak  ). These changes are most severe in the TEY mode, suggesting that the modification is strongest closest to the surface, consistent with the surface-sensitive wide-scan XPS in Fig. 5. The shift of spectral weight from the pre-edge
). These changes are most severe in the TEY mode, suggesting that the modification is strongest closest to the surface, consistent with the surface-sensitive wide-scan XPS in Fig. 5. The shift of spectral weight from the pre-edge  region to larger contributions between 534 and
region to larger contributions between 534 and  would be consistent with MnO formation initially upon heating,31 which we have shown is readily formed (Fig. 4). The emergence of the peak at
would be consistent with MnO formation initially upon heating,31 which we have shown is readily formed (Fig. 4). The emergence of the peak at 
 and increased spectral weight at
and increased spectral weight at  are also signatures of the O
are also signatures of the O  -edge of SrO.32 This interpretation would be consistent with previous XPS evidence of MnO and excess Sr in the quenched case. The TEY XAS of the quenched film therefore reflects a complicated mixture of both MnO and SrO species, and is most likely
-edge of SrO.32 This interpretation would be consistent with previous XPS evidence of MnO and excess Sr in the quenched case. The TEY XAS of the quenched film therefore reflects a complicated mixture of both MnO and SrO species, and is most likely  as proposed by la O' et al.7 The remaining La-deficient
as proposed by la O' et al.7 The remaining La-deficient  underneath this topmost layer would contribute stronger to the TFY mode signal, consistent with the larger pre-edge peak intensity at
underneath this topmost layer would contribute stronger to the TFY mode signal, consistent with the larger pre-edge peak intensity at  and the shoulder appearing at
and the shoulder appearing at  compared to the TEY mode XAS of the pristine and equilibrium cooled LSMO. As a result, the severe change in the local oxygen environment at elevated temperatures in air with no bias (i.e., initial stage of SOFC operation) observed in Fig. 8 can be understood in terms of various contributions of La-deficient
compared to the TEY mode XAS of the pristine and equilibrium cooled LSMO. As a result, the severe change in the local oxygen environment at elevated temperatures in air with no bias (i.e., initial stage of SOFC operation) observed in Fig. 8 can be understood in terms of various contributions of La-deficient  ,26 with a mixture of
,26 with a mixture of  ,7 and/or passive MnO (Ref. 31) and SrO (Ref. 32) species. Further analysis, such as density functional theory (DFT) calculations of O 2p PDOS of various
,7 and/or passive MnO (Ref. 31) and SrO (Ref. 32) species. Further analysis, such as density functional theory (DFT) calculations of O 2p PDOS of various  compositions and synthesis of
compositions and synthesis of  for comparison, is beyond the scope of this work.
for comparison, is beyond the scope of this work.
Figure 8. The O  -edge XAS of: (a) pristine LSMO; (b) heat-exposed LSMO cooled in thermal equilibrium; (c) heat-exposed LSMO rapidly quenched; and (d) rapidly quenched activated LSMO. Changes in the O
-edge XAS of: (a) pristine LSMO; (b) heat-exposed LSMO cooled in thermal equilibrium; (c) heat-exposed LSMO rapidly quenched; and (d) rapidly quenched activated LSMO. Changes in the O  -edge XAS spectra are indentified by arrows. The addition of a peak
-edge XAS spectra are indentified by arrows. The addition of a peak  in the highlighted hybridized Mn 3d–O 2p prepeak region (blue box) is shown.
in the highlighted hybridized Mn 3d–O 2p prepeak region (blue box) is shown.
Summary
We have demonstrated the ability to study the evolution of the Mn charge state, chemical composition, and electronic structure of LSMO cathodes from fabrication to after the activation with soft X-ray spectroscopy techniques. The problems associated with reaching SOFC operating conditions (i.e.,  , atmospheric pressure of
, atmospheric pressure of  , and
, and  cathodic bias) with conventional UHV-based spectroscopy techniques were circumvented by suitably quenching our LSMO thin films and employing a combination of XPS, XAS, and RPES techniques. Explicit care was taken throughout the study to ensure: (1) suppressed
cathodic bias) with conventional UHV-based spectroscopy techniques were circumvented by suitably quenching our LSMO thin films and employing a combination of XPS, XAS, and RPES techniques. Explicit care was taken throughout the study to ensure: (1) suppressed  formation associated with the surface preparation; (2) the ability of quenching to "freeze" the SOFC conditions; and (3) our dense (rather than porous) LSMO films displayed the effect of the enhanced catalytic activation or burn-in. Following the activation of the LSMO cathode, as confirmed by our corresponding EIS measurements, the O
formation associated with the surface preparation; (2) the ability of quenching to "freeze" the SOFC conditions; and (3) our dense (rather than porous) LSMO films displayed the effect of the enhanced catalytic activation or burn-in. Following the activation of the LSMO cathode, as confirmed by our corresponding EIS measurements, the O  -edge XAS revealed a dramatic change in the local oxygen environment in contrast to the Mn
-edge XAS revealed a dramatic change in the local oxygen environment in contrast to the Mn  -edge XAS, where only a reduced
-edge XAS, where only a reduced  presence near the surface was observed. These findings would initially support the hypothesis by la O' et al. , that
presence near the surface was observed. These findings would initially support the hypothesis by la O' et al. , that  formation plays a significant role in enhancing the performance of the device,7 along with the migration of
formation plays a significant role in enhancing the performance of the device,7 along with the migration of  species toward the LSMO/electrolyte interface (i.e., reduced
species toward the LSMO/electrolyte interface (i.e., reduced  species near the LSMO/air interface) as proposed by in situ photoemission microscopy measurements of LSMO cathodes by Backhaus-Ricoult et al.6 However, we highlight the need for DFT calculations to exactly determine the composition and confirm the presence of
species near the LSMO/air interface) as proposed by in situ photoemission microscopy measurements of LSMO cathodes by Backhaus-Ricoult et al.6 However, we highlight the need for DFT calculations to exactly determine the composition and confirm the presence of  rather than merely passive
rather than merely passive  and SrO formation.
and SrO formation.
In contrast to la'O et al. ,7 we observed severe changes in the chemical composition at elevated temperature even in the  atmosphere prior to activation with a cathodic bias. Wide energy range XPS surveys of rapidly quenched and vacuum sealed LSMO cathodes revealed significant La deficiency, severest near the surface region. The same studies of LSMO samples cooled in thermal equilibrium displayed relatively unchanged
atmosphere prior to activation with a cathodic bias. Wide energy range XPS surveys of rapidly quenched and vacuum sealed LSMO cathodes revealed significant La deficiency, severest near the surface region. The same studies of LSMO samples cooled in thermal equilibrium displayed relatively unchanged  ratios compared to the pristine samples. Meanwhile, the corresponding O
ratios compared to the pristine samples. Meanwhile, the corresponding O  -edge XAS spectrum of equilibrium cooled LSMO was similar to the pristine case reported elsewhere, and distinctly different from the rapidly quenched case. The change in the O
-edge XAS spectrum of equilibrium cooled LSMO was similar to the pristine case reported elsewhere, and distinctly different from the rapidly quenched case. The change in the O  -edge XAS spectra of burnt-in and heat-treated is considered to be due to the change in
-edge XAS spectra of burnt-in and heat-treated is considered to be due to the change in  ratio, which is also related to the formation of
ratio, which is also related to the formation of  species. Furthermore, the corresponding loss of the Mn 3d
species. Furthermore, the corresponding loss of the Mn 3d  state in the Mn
state in the Mn  -edge RPES, increased
-edge RPES, increased  contribution to the Mn
contribution to the Mn  -edge XAS, were explained in terms of increased hole doping due to the change in
-edge XAS, were explained in terms of increased hole doping due to the change in  ratio at elevated temperature in
ratio at elevated temperature in  . The absence of the
. The absence of the  state
state  in the photoemission spectra implies an insulating state is formed at elevated temperature and would suggest a hole doping of
in the photoemission spectra implies an insulating state is formed at elevated temperature and would suggest a hole doping of  in the remaining perovskite.40
in the remaining perovskite.40
Conclusion
In summary, the combination of passive  species near the surface and increased resistivity due to the hole doping
species near the surface and increased resistivity due to the hole doping  of the LSMO upon exposure to elevated temperature in
of the LSMO upon exposure to elevated temperature in  atmospheric pressures for SOFC operation is considered responsible for the initially poor performance of the device. The improved oxygen reduction following the application of a cathodic bias, as seen in our EIS measurements, is most likely related to enhanced bulk ORR kinetics due to the migration of
atmospheric pressures for SOFC operation is considered responsible for the initially poor performance of the device. The improved oxygen reduction following the application of a cathodic bias, as seen in our EIS measurements, is most likely related to enhanced bulk ORR kinetics due to the migration of  ions toward the LSMO/electrolyte interface and
ions toward the LSMO/electrolyte interface and  regions (formed initially upon elevated temperature) facilitating the reduction of the
regions (formed initially upon elevated temperature) facilitating the reduction of the  species.
species.
Acknowledgments
The BU program is supported by the U.S. Department of Energy grant no. DE-NT0004104. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under contract no. DE-AC02-05CH11231. Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract no. DEAC02-98CH10886. A portion of the research was performed using EMSL, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory.
Boston University assisted in meeting the publication costs of this article.