Abstract
Electrochemical biosensors have attracted a tremendous attention for many researchers recently due to its facile synthesis process, tunability easiness by tailoring the material properties or composition, and wide range of biological analyte types detection. To obtain an excellent electrochemical biosensor performance, a material that facilitates fast electron transfer, large surface area, excellent electrocatalytic activity, and abundant available sites for bioconjugation is immensely needed. Metal-organic frameworks in the two-dimensional form (2D MOFs) provide all of the criteria needed as the sensing material for electrochemical biosensors application. However, the design and preparation of 2D MOFs, which have high stability and sensitivity as well as good selectivity for biological analyte detection, is still quite challenging. This review provides the recent studies and development of 2D MOFs as electrochemical biosensor. A detailed discussion about 2D MOFs structures, their synthesis strategy and control, 2D MOFs materials in electrochemical biosensor application, and the future challenges is thoroughly explained in this review. Hopefully, this review will also provide a new inspiration to advance future studies of 2D MOFs materials development as electrochemical biosensor.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Two-dimensional (2D) materials have become a popular research topic for material research communities, especially for their applications as an electrochemical biosensor. This is because 2D materials have unique morphological structures characteristic, such as a large surface area, fast electron and mass transfer, and excellent electrocatalytic activity, which allow them to have many advantages as an electrochemical biosensor. In their electronic properties specifically, 2D materials have various of energy bands which can be different with their bulk form counterpart.1 This energy band structures variation allows them to have many types, i.e. insulator, semiconductors, semimetals, metals, and superconductors.2 In terms of the materials composition, 2D materials can be categorized into several groups, i.e. graphene and graphene oxide, elemental nanosheets, transition metal oxide, transition metal dichalcogenides, MXene,3–7 group-VA semiconductor, graphitic-carbon nitride,8 hexagonal boron nitride,9 layered oxides, layered double hydroxides, layered zeolites, and 2D polymers.10,11 These unique properties along with the large surface area that they have provide them to have an extensive interaction with the external environment and making them to be much favorable over the bulk counterpart for sensing application.12
On the other hand, researchers also have a considerable interest in developing metal-organic frameworks (MOFs) because of their high thermal and chemical stabilities that lead to an excellent electrochemical biosensor performance.13 MOFs are a type of materials that can also be described as a porous coordination system built by metal and organic ligands in a unique coordination.14,15 Furthermore, the morphological characteristics of MOFs can be improved by modifying the metal-ligand coordination. Interestingly, this improvement can also be observed from their chemical, physical, and biological properties.16 Since the structure can be easily altered by changing the metal-ligand combination, the MOFs structure can be expanded from 1D to 2D or 3D form. In 2D form, MOFs have become a new exciting research topic because of their ultrathin thickness and its abundance of active sites.17 Moreover, their micro/nanocrystals surface energies can also affect some essential characteristics, i.e. thermodynamics and kinetics processes, including the reaction on the interface and the nanocrystals formation. 2D MOFs have also good thermal and chemical stability characteristics.18 Based on these advantages, 2D MOFs are very potential to be applied in various applications, such as gas separation, energy storage and conversion, catalyst, biomedicine, and biosensors.19
Biosensor is a type of sensor that can identify the type of a biological analyte and/or quantify its concentration. The biological analytes discussed here, include biochemical compounds (e.g. dopamine, ascorbic acid, glucose), a strain of nucleic acid (RNA or DNA), an antibody, a virus particle, and so on. There are currently several biological analyte detection methods, including enzyme-linked immunosorbent assays (ELISA),20 chemiluminescent assay,21 radioimmunoassay,22 and electrochemical assay.23 Among these methods, electrochemical based detection attracts much interest due to its easy operation, simple analytical method, and easy preparation.24 Electrochemical biosensors assays also provide both qualitative and quantitative detection that are very needed in medical diagnosis and monitoring therapy of the disease that cannot be achieved by conventional assay. In electrochemical biosensor applications, to achieve a good performance, the materials are required to have an abundance of active sites. By using 2D MOFs as the sensing platforms, the performance of electrochemical biosensors could be significantly enhanced due to the excellent electrochemistry properties of the 2D MOFs. Thus, porous 2D MOF becomes a promising candidate for a superior electrochemical biosensor material. In porous 2D MOF, interactions with ions or molecules occur not only on the surface, but also in the depth section of the materials. Furthermore, inherited by the properties of 2D materials, some advantageous characteristics of 2D MOFs for electrochemical biosensor applications, include: (1) having a well-defined pore structure, which can encapsulate external molecules, so that the electron transfer increases.25 (2) having a better sensitivity because of their excellent electronic characteristics.26–29 (3) having an excellent physicochemical properties, which include good biocompatibility, highly specific structural area, and strong interaction with biomolecules, proteins, DNA, cells, and other bio-organisms on their interface.29,30 The enzymes in bio-organisms are easily attracted to MOF, enabling 2D MOF to have a good catalytic performance.31,32 Thus, it is expected that MOFs in 2D form can provide a highly sensitive detection system for electrochemical biosensor applications.33
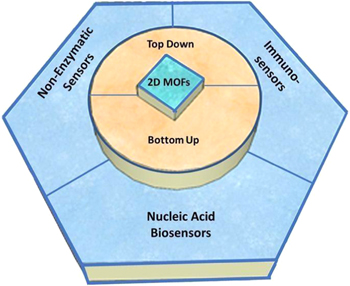
Overall, this article provides a comprehensive review on the basic structures of 2D MOFs as well as the opportunities and challenges of 2D MOFs for electrochemical biosensor applications. The organization of this paper is illustrated in Fig. 1. Firstly, we review various MOFs composition groups along with their synthesis methods, including the top-down and bottom-up approach, and also the procedure on how to obtain 2D MOFs with a high-performance electrochemical biosensing properties. Then, 2D MOFs for electrochemical biosensors such as non-enzymatic sensors, nucleic acid biosensors, and immunosensors are discussed. Finally, we provide the outlook of the 2D MOFs potentials/challenges in the future as an electrochemical biosensor.
Figure 1. The organization of this review article on describing 2D MOFs for electrochemical biosensor applications.
Download figure:
Standard image High-resolution image2D MOFs Structures
Monometallic 2D MOFs
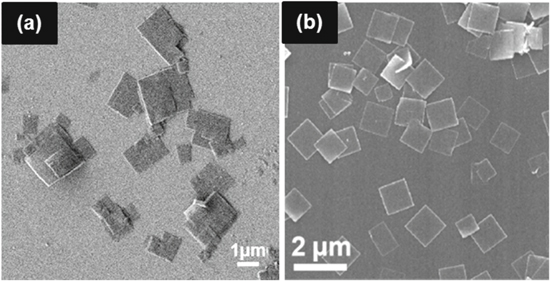
Monometallic 2D MOFs generally consist of single metal and organic ligands. Their structures and characteristics depend on the selection of metal and ligand in the desired formation. Liu et al. prepared two types of 2D MOF monometallics using ultrasonic assisted precipitation methods. The MOF comprised of Nickel (Ni) or Cobalt (Co) metal-ion and H2BDC (1,4-benzenedicarboxylic acid) ligands. The abbreviation of these MOFs are NiBDC and CoBDC. Both MOFs had similar structure of nanosheets with the thickness of 3 nm that was resulted from three or four coordination layers. Their morphological structure is shown in Figs. 2a and 2b.34 Zhao et al. had synthesized another type of 2D monometallic MOF with a Cu metal center. The MOF had a nanosheet structure in the shape of a square lamellar, as shown in Fig. 2c, with the thickness in the range of 5–25 nm and a lateral size of 0.5–4 mm. This structure is related to the nucleation and crystal growth by the slow diffusion process in the intermediate layer. Continuous diffusion of Cu2+ and BDCA linker to the middle layer produces more Cu-BDC nanosheets.35 Co-BDC has similar coordination with its Ni counterpart. The metal is octahedrally bonded with six oxygens from BDC ligands and each metal is connected to each other in the certain plane direction forming metal-oxygen layers.36,37 The layer then bridged to other layers by BDC ligands in another direction. In the case of Cu-BDC, the MOF consisted of two 5-coordinate copper cations in which they covalently bond to each other.38 Each pair of copper bridged with four BDC ligands to other pairs forming a 2D framework.
Figure 2. Monometallic 2D MOFs structures with various types of metal ions: (a) 2D Ni-BDC (b) 2D Co-BDC Reprinted from publication34 with permission from Elsevier (c) CuBDC MOF nanosheets Reprinted from publication35 with permission from Elsevier.
Download figure:
Standard image High-resolution imageBimetallic 2D MOFs
Bimetallic 2D MOFs are produced by combining two metals at a certain ratio or substituting a few metal atoms with another metal atom. The resulting structure of 2D MOFs highly depends on the ratio of the two metals. The incorporation of second metal aims to modify the structure and electronic properties of 2D MOFs.39,40 Ma et al.41 found that the enhancement of electrochemical properties of NiCo–MOF was caused by the partial electron transfer from Ni2+ to Co2+ through oxygen in the BDC ligand. Moreover, the synergetic effect of NiCo bimetal MOF also contributed to its high electrochemical activities. The corresponding structure and SEM images of Ni–Co MOF are shown at Fig. 3. However, Li et al. reported a slightly different result. In their NiCo–MOF, the electron transfer was from Co to Ni as observed from the X-ray Photoelectron Spectroscopy (XPS) measurement.42 Another study by Hao et al. reported that adding Fe atom to Ni–MOF system nanosheets, in the case of NiFe–MOF, could significantly improve the kinetics of the charge transfer reaction.43
Figure 3. (a) Schematic illustration of the synthesis process of bimetallic 2D MOFs (NiCo–MOF nanosheets array), (b) SEMand (c) TEM images of the NiCo MOFs nanosheets Reprinted from publication42 with permission from Elsevier.
Download figure:
Standard image High-resolution imageFurthermore, Zhou et al. had successfully synthesized 2D bimetallic ZnZr–MOF with a new strategy that is MOF-on-MOF method. The structure displayed the bonding between Zn–MOF nanosheet and Zr–MOF layer. The report also found that the order of metal precursors and organic ligands addition would affect chemical structure, crystalline properties, and surface functionality of 2D bimetallic ZnZr–MOFs. The order of adding metal precursors is the difference in the process of forming Zn-MOF-on-Zr-MOF or Zr-MOF-on-Zn-MOF. This significantly affected different structure and characteristics of MOFs. In detail, Zn-MOF-on -Zr-MOF will have better sensing properties than Zr-MOF-on-Zn-MOF-, Zn-MOF-, and Zr-based sensors. This is because Zn-MOF-on-Zr-MOF has a stronger bioaffinity and higher stability. This can be explained by the Zr–MOF substrate that assists the penetration of Zn (II) ions into the Zr–MOF node, which resulting in complete integration of Zn–MOF and Zr–MOF. Meanwhile, Zr (IV) ions cannot easily penetrate the Zn–MOF interior because of the small pore size, so that Zr–MOF will cover the surface of Zn–MOF. Zn-MOF-on-Zr-MOF has a unique flower-like structure with a size around 10 ± 2 μm and has no clear lattice spacing, whereas Zr-MOF-on-Zn-MOF has a similar structure to pure Zr–MOF with identical lattice fringes are widely distributed.44 Another study showed that the addition of Zn to the NiZn–MOF nanosheet could adjust the porosity and electronic structure of the nanosheet. The NiZn–MOF had a flower- like structure assembled from nanosheets providing a high specific surface area and a fast electron/mass transfer channel. This fast charge transfer might be resulted from the synergistic interaction of two metal ions in this material.45
Generally, the stability of MOFs depend on the metal nodes chemical stability that is strongly influenced by the properties and the environment of metal nodes itself, such as rigidity, size of the organic ligands that are connected to metal nodes, and the number or type of surrounding metal nodes interconnected via organic ligands. As an example, in bimetallic NiCo–MOF (NiCo(-BTB)4(bipy)3), not only the Ni-based, but also the Co-based paddle wheel SBUs (secondary building units) interconnects via the organic ligands. This coordination have a more stable framework as compared to the pure Ni–MOF.46 The stronger interaction of the metal-ligand bond will protect the MOFs against hydrolysis. Moreover, in the mixed metal or bimetal MOFs, replacing or combining metal nodes with a less valent metal ion will destabilize the final MOFs product, which resulting in a charge deficit that needs to be compensated by exchangeable cations.47
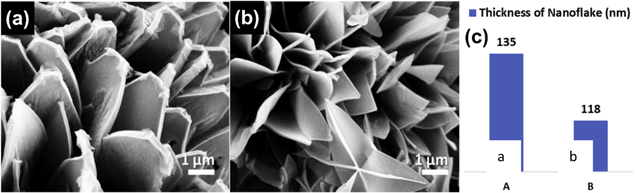
The morphological structure of the bimetallic 2D MOFs also depends on the ratio of the two metals themselves. Y. Hao et al. synthesized Ni–BDC, Fe–BDC, and Ni0.75Fe0.25-BDC, then observed the morphology transformation after Fe addition. The Ni0.75Fe0.25-BDC exhibited 2D structure that was adapted from Fe–BDC. This transformation caused more efficient catalytic performance of 2D MOF and also improved its stability and electrochemical properties. The Other Ni:Fe ratios such as Ni0.5Fe0.5-BDC and Ni0.25Fe0.75-BDC, had a similar structure to the raw ratio.43 In addition, Lim et al. also found that the addition of Zn could modify the structure of Co–MOF and significantly reduced the thickness of nanoflakes, as shown in Fig. 4. This reduction in thickness allowed a reduction in resistance and provided a larger surface area helped improving the electrochemical performance.40
Figure 4. SEM images of Zn that caused the thickness reduction of the nanoflakes. Reprinted from publication40 with permission from Elsevier.
Download figure:
Standard image High-resolution imageAlongside the number of metal ions, the types of ligands also influence 2D MOFs structures. Ligands in 2D MOFs can be in the form of single-type ligands or mixed-type ligands which are further explained in the following sections.
Single-types ligand
2D MOFs can be synthesized using one type of ligand which has two or more binding groups and positioned at the appropriate coordination. The types of ligand commonly used in 2D MOFs are aliphatic organic ligands including hydroxy acetic acid, diacetic acid, and adipic acid.17,48 Ligands with high symmetry (e.g. C3, C4, D3h and D4h) are commonly used to design 2D MOFs. The C3 and D3h symmetrical ligands are used to form 2D MOF that extend in the equilateral triangular direction to design 2D MOF formation. Those symmetrical ligands have a lot of attention because of their ability to form 2D MOF with a good conductivity. Different results will be obtained if 2D MOF preparation employs D4h symmetry ligands. Coordination with metal will cause the ligand to extend in quadrilateral direction. It has been reported that the D4h symmetry ligand is popular to be used to prepare 2D MOF due to its predictable structure and viability to afford metal doping, especially for D4h ligand that contain porphyrin.17 In addition, there are also aromatic ligands with carboxylic groups, such as 1,4-benzene dicarboxylic acid and 2,2'-dithiodibenzoic,49 and N-heterocyclic rings, such as pyridine. Both are preferred for the preparation of 2D MOF because of their rigid structure and fixed directional angle that make the 2D MOF structures become simpler.50–53
B. Liu et al. successfully synthesized 2D Co-MOF using 1,4-benzene dicarboxylic acid (BDC) ligand with bottom up method resulting a uniform two-dimensional thickness and exposed metal atoms. This structure had an impact on a faster and more stable electron transfer. As well-known, the attractive characteristics of the 2D MOFs are their interconnected porous structure and high surface area, which can increase electron transfer and mass diffusion. In this case, the Co ion is effectively coordinated with 1,4-BDC ligand,34 In most cases, BDC ligands are used to configure 2D MOF because of the ability to form 2D structures very well.54,55 Moreover, the modification of BDC ligand with –NO2 group may affect the electron density of the dicarboxylic ligands. Therefore, different physical and chemical properties of MOF can be obtained.56
Mixed-types ligand
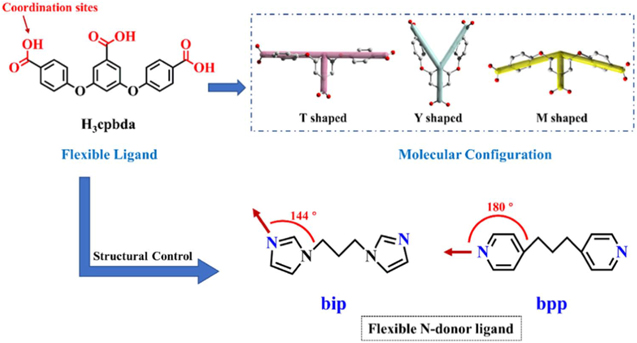
2D MOF with single-type ligands has limited possible coordinate angles due to the limited number of ligands suitable to form 2D MOFs. This limitation results in a reduced option to tune the characteristic of the 2D MOFs, knowing that the various features of MOF are due to their numerous combination of metal-ion and ligands.17,57 These drawbacks can be solved by adding more ligands or commonly called as the mixed-types ligand. Lei Wang et al. reported a combination of 4,4'-bipyridine and 1,2,4-benzene tricarboxylate ligand in coordination with Cu metal.58 In their case, 2D structure was constructed by Cu2(CO2)4(4,4'-bpy)2 paddle-wheel like as a secondary building unit (SBU) and 1,2,4-BTC as a linker. X.F. Yang et al. also reported the synthesis of 2D MOF nanosheets, [Cu(Hcpbda)(bpp)(H2O)]n, by using two different ligands, H3cpbda and 1,3-bis(pyridin-4-yl)propane (bpp), with the illustration of the ligand structures are shown in Fig. 5 and the comparison of the crystalline structure at single and mixed type ligand is shown in Fig. 6. Deprotonated Hcpbda2− ligand coordinated with Cu cations forming 1D chains. The bpp ligands acted as a bridge that connect each Cu cation in another direction. They also reported some possible coordination of multi carboxylic acid with different N-donor ligands to produce a two-dimensional MOF.59 They concluded that the coordination angles, the ligand length and the size of the rigid ligand affected the 2D structural construction. N-donor ligands, such as ligands that contain pyridine and imidazole, are more developed because of their ability to be the excellent bridge to produce attractive MOF structures.60 O-donor ligands, such as polycarboxylate and naphthalene disulfonate, offer stable coordination in MOF formation due to their ability to strongly bind with the metal center.60,61
Figure 5. Illustration of flexible ligands structure (H3cpbda, bip and bpp) in forming mixed-type ligand MOF. Reprinted from publication59 with permission from Elsevier.
Download figure:
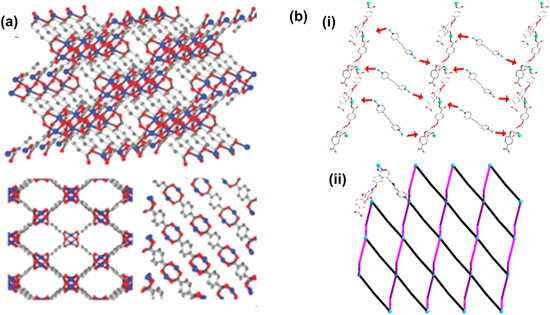
Standard image High-resolution imageFigure 6. Illustration of crystalline structure of (a) 2D CuBDC Reproduced from Ref. 62 with permission from the Royal Society of Chemistry. (b) [Cu(Hcpbda)(bpp)(H2O)]n (i) Hcpbda2- and Cu2+ ligand connect the 1D chain (ii) bind to bpp ligand along the a axis, the 2D sheets that are formed can be simplified as (4,4) –net. Reprinted from publication59 with permission from Elsevier.
Download figure:
Standard image High-resolution imageLayered MOF can be achieved by combination of two or more ligands to get entanglement phenomena. Entanglement is an interesting phenomenon in MOF that are constructed by longer organic ligands, such as 1,4-bis(benzimidazole-1-yl)butane (bimb) and the rigid 1,6-naphthalene disulfonate (1,6-NDS).60 It produces large voids in its network. Some examples of entanglement systems are interpenetration, polycatenane, polyrotaxane, polythread, interdigitation and polyknotting. Zhao et al. constructed the [Cd(Seb)(1,4-mbix)] entanglement MOFs with the aliphatic dicarboxylic acid (Seb) as the host ligand and two isomeric bis(2-methyl-imidazole) ligands (1,4-mbix) as the auxiliary ligands and Cd as a metal ion. The 1,4-mbix ligand and the aliphatic dicarboxylate anion ligand were in parallel binding to form a 2D-2D interpenetration network. The Cd (II) ion had the same six-coordination that bonds with two nitrogen atoms of the two 1,4-mbix ligands and four oxygen atoms of the two anionic Seb2− ligands established an octahedral geometry. These [Cd2 (1,4-mbix)2] rings were linked by Seb2− (O1 and O2) along a direction as a linear linker without penetrated into the ring [Cd2 (1,4-mbix)2] and another Seb2− (O3 and O4) along c direction as a rod that penetrated into the ring [Cd2 (1,4-mbix)2] of the other layers. These produced two identical 2D single network that would be linked to each other in parallel, resulting in a 2D + 2D → 2D interpenetration layer by polyrotaxane feature.63 Figure 7 shows the SEM images of single ligand and mixed-type ligand 2D MOFs.
Figure 7. (a) SEM images CuBDC MOF nanosheets. Reprinted from publication64 with permission from Elsevier. (b) SEM image of PPF-3 nanosheets (mixed ligand of 5,10,15,20-tetrakis(4- carboxyphenyl)porphyrin (TCPP) and 4,4'-bipyridine (BPY) Reprinted with permission from Ref. 65. Copyright 2016 American Chemical Society.
Download figure:
Standard image High-resolution imageSynthesis Methods of 2D MOFs
Generally, 2D MOFs can be prepared by two synthesis methods, i.e., top-down and bottom-up methods.66 A more detailed description of the top-down and bottom-up method is explained in the following sections.
Top-down
A top-down method is a synthesis approach that is performed as an attempt to exfoliate the bulk layered-materials.67 This exfoliation process is the disintegration process of MOF crystals into single or multilayer structures which can easily occur due to the existence of noncovalent interactions that involve hydrogen bonding, π-π interactions, and van der Waals forces.23 The exfoliating of bulk layered material can be advantageous for synthesizing 2D materials because it can produce an exfoliated 2D material with a simple and low-cost procedure. Moreover, many layered-material types can not be synthesized using other methods except exfoliation. The exfoliated 2D material can also be processed in solution, which may facilitate their fabrication into desired structures and applications.67 There are several methods to do exfoliation, such as sonication exfoliation, shaking treatment, ball-milling, mechanical exfoliation, and intercalation or chemical exfoliation.68 Mechanical exfoliation is the commonly used synthesis method for the top-down method since it can produce 2D MOFs with good crystallinity and purity.69 However, it should be noted that exfoliation requires multistep processes, which may cause the layer interactions to be weakened.70
Sonication exfoliation
Sonication exfoliation is a simple and effective method to synthesize ultrathin single-layer 2D nanomaterials. This exfoliation utilizes sound waves to agitate particles in solution. In this context, sonic waves are used to break the bonds between layers of bulk MOF, so that 2D MOF can be obtained. Currently, this approach has been developed by many researchers. For instance, in the earlier 2015, Alavi et al. had successfully used the sonication approach to exfoliate {[Cu2(BDC-NH2)2(dabco)]DMF.3H2O}. They found that sonication time dramatically influenced the quality and the distribution of the nanostructures. They claimed that 60 min is the optimum time of the sonication process because it gave the materials' best structure. It was confirmed that 30 min of sonication duration was not enough that the structures of 2D MOF did not grow. When the duration of sonication was longer than 60 min, 90 min and 120 min, the 2D MOF structures had agglomerated.71
Two groups of researchers also successfully applied sonication exfoliation in their research. They claimed that a suitable solvent in the sonication process is water. Firstly, In earlier 2019, Wu et al. synthesized 2D Cu–MOF nanosheets using a sonication exfoliation approach for 20 min in water. In their work, they confirmed that the successful exfoliation result using TEM and atomic force microscopy (AFM). Based on the TEM analysis, the MOF nanosheet showed its ultrathin nature. Meanwhile, the AFM image showed that the MOF nanosheet had a uniform thickness of 3.4 nm.72 Moreover, in early 2020, Pang et al. used this approach to obtain 2D MOF nanosheets. ([M2(bdc)(dabco)] (M = Zn, Co, Ni, H2bdc = 1,4-benzene dicarboxylic acid, dabco = 1,4-diazabicyclo-[2.2.2]octane)) was subjected to sonication in water. The exfoliation process provided an ultrathin layer of 2D nanomaterial (0.9 nm).73 It was confirmed that the MOF nanosheet had a good stability in the water at the sonication process.
Shaking treatment
In some special cases, bulk MOFs have been delaminated using a shaking treatment. Araki et al. successfully did this approach. in 2013 they synthesized La(BTP) (BTP = 1,3,5-benzene triphosphonic acid) using a shaking treatment. They produced nanosheets with approximately 1.3 nm of thickness.74 A similar result was also reported by Kondo et al. in 2013. They synthesized the ultrathin 2D [Cu(bpy)2(OTf)2] (named CuMOF) (bpy = 4,4'-bipyridine; OTf = trifluoromethanesulfonate). In their research, MOF nanosheets were obtained by shaking treatment in acetone. Similar to the sonication approach, shaking treatment also depends on the duration of the treatment. Kondo et al. had investigated the effect of treatment duration on the 2D structure. The duration was varied to 1 h, 3 h, 6 h, and 12 h. They found that 1 h and 3 h of the treatment resulted in thick MOF sheets. Meanwhile, the 6 h shaking treatment resulted 2D CuMOF nanosheets to have a thickness of 4–5 nm based on the AFM characterization. Lastly, the 12 h shaking treatment could break 2D CuMOF into small pieces with a size of less than 500 nm. They concluded that the best duration treatment is 6 h because it gave a smooth surface with a sufficient thickness.75 Based on these results, we know that the duration time has an important role in shaking treatment.
Ball-milling
This approach is relatively new for synthesizing 2D material and still has limited in the heterogeneities of the product, moderate porosity, and limited crystallinity. Several researchers have successfully performed this approach. In 2014, Peng et al. synthesized Zn2(bim)4 (bim = benzimidazole) using a ball milling approach. They found that the final MOF crystals had interlayer distance of approximately 0.988 nm.76 In 2019, another study about ball-milling had been reported by Yoon et al. who synthesized the bimetallic conductive 2D metal-organic framework (Co0.23Ni0.77-CAT). Ball-milling processes produced a relatively thick lateral layer with approximately 1.91–2.21 nm that was confirmed by HRTEM characterization.77
Mechanical exfoliation
Mechanical exfoliation is one of exfoliation technique that gives a high possibility to produce a good quality of a few-layer nanocrystals.78 Currently, 2D material samples which synthesized by mechanical exfoliation have an unsurpassed quality in terms of purity.79 Zhu et al. (2019) stated that mechanical exfoliation can exfoliate the bulk crystal using plastic tape and can be used in almost all 2D materials, including 2D MOFs.80 In 2018, López-Cabrelles et al. reported that they had successfully exfoliated [Fe(bimCl)2] (HbimCl = 5-chlorobenzimidazole) using this method. Their research declared that the material had a layered structure with the packing thickness of approximately 400 μm.81
Intercalation or chemical exfoliation
Chemical exfoliation from MOFs potentially allows efficient and controllable formation of 2D MOF nanosheets. This approach was reported by Ding et al. in 2017. They demonstrated the chemical exfoliation approach to obtain MOF nanosheets from intrinsically layered MOF crystals. They claimed that the chemical exfoliation process could proceed at room temperature. The thickness of the produced 2D MOF nanosheets was approximately 1 nm, with an overall yield of 57%.82 In another case, the delamination procedure was applied to Cu–BDC bulk to obtain 2D Cu–BDC. Generally, the coordination of Cu–BDC was formed by the coordination of Cu ion and H2BDC in planar geometry. Each layer was connected by DMF molecules in the a-axis direction, forming stacked layers.83 Post-treatment of Cu–BDC by immersing it in the ethanol solution containing amino compounds was reported could delaminate the stacked layers. This post-treatment procedure did not affect the crystal structure of Cu–BDC.
Bottom-up
Similar to the top-down method, the bottom-up method also has several types of synthesis processes such as interfacial synthesis, three-layer synthesis, surfactant-assisted synthesis, and modulated synthesis.68 Bottom-up method is considered as the most promising method that can produce more applicative and potential material. However, 2D MOF nanosheets that are synthesized using this method are sometimes still relatively thick, around 5–50 nm, and the production process is limited. Therefore, the large scale synthesis process using this method remains a challenge.84 Although the bottom-up method allows a direct growth-ensuring, the morphological structure and thickness of 2D MOF can be entirely controlled. However, the addition of foreign substances will inevitably lead to an inadequate utilization of active sites on the surface.85 The bottom-up method's critical point is to limit the vertical growth direction of the 2D MOF layer due to the strong interaction between each layers to achieve the lowest surface energy.86 Hence, some of the researchers also used the bottom-up method to synthesize 2D MOFs, especially the interfacial synthesis approach.87 Interfacial synthesis is generally a liquid-liquid or liquid-air interfacial reaction.88
Interfacial synthesis
This approach depends on the reactant's dispersive behaviors in two separate phases, which could control the diffusion of the reactants from one phase to the other phase.89 This method is one of the most widely used of the bottom-up methods to synthesize MOF nanosheets. This method is often used with several approaches, such as liquid/liquid interface or liquid/air interface in controlling the growth of MOF nanosheets. For instance, the synthesis of nickel bis(dithiolene) nanosheets was successfully reported by Kambe et al. in 2013. They achieved the synthesis by reaction of nickel(II) acetate and benzenehexathiol at liquid-air interface. Their research was done by spreading a thin layer of ethyl acetate solution that contained benzenehexathiol to the surface of aqueous solution that contained nickel(II) acetate. The evaporation of ethyl acetate in the liquid-air interface formed the nanosheets. Then, the nanosheets were investigated using scanning tunneling microscopy and it was observed that the hexagonal pattern of single-layer nanosheets had a height of 0.6 nm.90
Three-layer synthesis
This approach was conducted by Rodenas et al. (2015) in order to synthesize 2D MOF Cu–BDC. Rodenas et al. synthesized the Cu–BDC in a glass test tube. This method uses three layers which are differentiated by density. The top layer is the metal precursor solution and the lowest layer is the H2BDC ligand solution with the middle layer is the intermediate solvent layer. Under static conditions, the diffusion of metal cations and BDC ligands into the intermediate layer causes the growth of MOF crystals to occur locally in a highly diluted medium. This method requires no immiscible liquid phase, as opposed to the interfacial method, in which the interface rate determines the MOF growth. Their research produced a 2D MOF CuBDC with a lateral dimension of 0.5–4 μm and the thicknesses in the range of 5–25 nm.54
Surfactant-assisted synthesis
This approach not only controls the MOF growth in the vertical direction, but also improves the dispersion of MOF nanosheets in the liquid phase.68 This approach had been successfully reported by Wang et al. in 2020. Their research reported a sodium dodecyl sulfate (SDS)-assisted solvothermal to produce 2D c-MOF nanosheet including HHB-Cu (HHB = hexahydroxytriphenylene), HHB–Ni and HHTP–Cu (HHTP = 2,3,6,7,10,11- hexahydroxytriphenylene). The SDS surfactant was used to direct the MOFs' anisotropic growth in the 2D direction and resulting in the formation of ultrathin and large-sized nanosheets. SDS had hydrophobic chains that could weaken the interactions of interlayers and disassembled it to form ultrathin nanosheets during the sonication treatment. Moreover, it prevented further agglomeration by helping to stabilize the as-synthesized HHB–Cu MOF NSs in solution. The AFM measurement revealed a thickness of 4.5 ± 1.4 nm.91
Modulated synthesis
Apart from surfactants, several small molecules (e.g. acetic acid, pyridine) have also been used in the synthesis of the MOF nanosheet. These molecules, also known as modulators, have the same functional groups as organic bridges, which competitively coordinate with metal nodes to regulate the growth kinetics of MOFs. More importantly, the modulator's selective coordination in a particular crystal plane would inhibit the growth of MOFs along that direction, leading to the anisotropic growth of MOF with different morphologies. Two groups of researchers have reported this approach. Firstly, in 2017 Hu et al. successfully applied this approach to obtain 2D layered MOF nanosheets, NUS-8(Zr/Hf). These 2D layered MOFs were composed of Zr6O4(OH)4 or Hf6O4(OH)4 clusters and 1,3,5-benzene tribenzoate (BTB−3). Examination by field-emission scanning electron microscopy (FE-SEM) strongly indicated that the 2D layered nanosheets had been formed. It exhibited the nanosheets morphology with a thickness of 10–20 nm and the lateral size of approximately 500–100 nm.92 Secondly, in 2019 Nian et al., also synthesized 2D cobalt-based MOF CO2(bim)4 (bim = benzimidazole) nanosheets. Their research found that the lateral size of the 2D MOF nanosheets was up to 800 nm–3 μm. The SEM characterization revealed that the layered had a thickness of approximately 30 nm.86
As we know, both bottom-up and top-down synthesis methods can produce MOFs in two-dimensional form. However, according to several studies top down methods based on exfoliation have a chance of restacking 2D layered MOFs to the bulk form after volatilizing the solvent. This will be a limitation of the application in electrochemical biosensors.62 The obtained 2D MOFs from the exfoliation synthesis approach also display low stability against aggregation that will reduce the catalytic performance in electrochemical sensing. Thus, we suggest that it is better to use a bottom-up strategy to obtain 2D MOFs for electrochemical applications. Moreover, bottom-up approaches such as layer by layer synthesis or surfactant-assisted synthesis allowed a controllable MOF structure and properties such as vertical orientation that facilitates more active surface area and interlayer spacing that provide additional channels for ion transport, leading to the increased sensing performance.
Besides, the ultra-large surface area of 2D MOFs can be obtained from both top-down and bottom-up synthesis process. MOFs in two-dimensional form also promisingly have high mechanical stability and optical transparency that give a lot of benefits in the development of flexible and transparent biosensors devices in the future. Moreover, the ultra-thin thickness layer of 2D MOFs also allows fast electron mobility to the electrode surface and provides a high surface to volume ratio that facilitates easier contact between the biological analyte and the active sites.
As well explained in the previous paragraphs, the top-down and bottom-up methods have their own benefits and also drawbacks. These are summarized in Table I. Additionally, the summary of various 2D MOFs synthesized using the top-down and bottom-up method is provided in Table II.
Table I. The benefits and drawbacks of top-down and bottom-up method for synthesizing 2D MOFs.
| Method | ||
|---|---|---|
| Top-down | Bottom-up | |
| Benefits | 1. Some materials that cannot be synthesized or very challenging to be synthesized using the bottom-up or chemical method are very easy to synthesize using the top-down exfoliation method.67 | 1. The bottom-up method can be used to synthesize MOF in the form of nanosheets with the desired thickness.93 |
| 2. The top-down method can achieve a scalable production of 2D materials because the layer precursors are available in large quantities.67 | 2. The bottom-up method that is conducted using solution-based can produce MOFs nanosheets with a good performance.93 | |
| 3. The 2D material can be synthesized for a particular desired structure and application.67 | 3. The bottom-up method can be used for synthesizing non-layered MOF nanosheets.93 | |
| Drawbacks | 1. The top-down method is only suitable for MOFs which has layered structure.94 | 1. Bottom-up has limited interface area and it causes the yield of MOF nanosheets are low.93 |
Table II. Various 2D MOFs synthesized using top-down and bottom-up methods along with their application as a biosensor.
| 2D MOFs | Method | Application | References |
|---|---|---|---|
| Ni–Co–BDC | Top-down | Non-enzymatic H2O2 sensors | 34 |
| Co–BDC | Top-down | Non-enzymatic H2O2 sensors | 34 |
| Ni–BDC | Top-down | Non-enzymatic H2O2 sensors | 34 |
| Ni–Co–H2BDC–NH2 | Bottom-up | Electrochemical glucose sensing | 42 |
| H2BDC–NH2 = (2-amino-terephthalic acid) | |||
| Cu–TCPP | Bottom-up | Electrochemical ratiometric sensor for dopamine detection | 95 |
| TCPP = tetrakis (4-carboxyphenyl)porphyrin |
Ligand design
The ligand design formed in the MOF synthesis also provides the strength in the MOF assembly procedure. Topological predictions with the geometric predictability of the ligands making the constituent materials excellent in the MOF synthesis process. The ligand design aims to enrich the MOF topological types and modify the MOF functionality to be applied in certain applications. For example, some carboxylic ligands have been designed for both synthesis convenience and crystal discomfort purposes. The various ligand designs will also have various functions and also the collection of these ligand designs systematically makes it easier to synthesize according to the desired functionality.96
Substitution
the substitution process has been identified as a new strategy to obtain the desired MOF material. This substitution process is expected to increase the controllability of the MOF material both in terms of structure and properties. The parameters can be controlled for the substitution. For example, the concentration of reactants or reaction time. This process was carried out to obtain the substituted product as desired, either partial or complete substitution. Either partial or complete substitution involves cleavage and regeneration of the coordination bond between the metal ion and the organic ligand. Partially substituted products usually produce unique functionality. On the other hand, the substitution process also can be carried out on metal ions. This process is usually observed in materials that are less chemically stable, namely the coordination bond between the metal and the ligands which are relatively unstable. In this context, the substitution reaction is expected to be an ideal approach in the process of synthesis and modification of material properties including MOF.97 For example, in 2D MOF this process was investigated by Chen et al. in 2015. They investigated the effect of substitution of coordinated metal ions, e.g. from Ni to Cu in the MOF, on the structural and electronic properties of the MOF bulks and 2D sheets. With substitution of the metal sites, the band structures of the Cu3(HITP)2 sheet near the fermi level became quite different from those of Ni3(HTIP)2.98
Layer by layer
In the MOF deposition process, layer by layer is considered an important approach for depositing films on various types of substrates. In this approach, a solution containing a source of metal ions and organic ligands is mutually supplied to the crystalline growth surface to allow the growth of MOF with a thin thickness and a well-controlled surface. This approach shows the effective modulation technique in controlling crystal morphology. The main advantage of using this approach is having the precision to control the film thickness up to the molecular scale.99
2D MOFs for Electrochemical Biosensor Applications
Another classical obstacle for metal-organic frameworks (MOFs)'s application in electrochemical biosensors is the poor dispersibility of MOFs in solution due to the micrometres size of the bulk MOFs. This problem causes MOFs to be easily detached off the surface of the electrode during measurement.100 The nanoscaled and ultra-thin MOFs layer is required to solve this problem. Among all MOFs that are being developed, 2D MOF nanosheets become the widely explored due to their unique characteristic and many advantages such as large surface area and very thin thickness which results in an excellent electrocatalytic activity, a large number of accessible sites for biomolecular adsorption, and most importantly good dispersibility in solution.101 Here, the new research direction of 2D MOFs for electrochemical biosensor, as illustrated in Fig. 8, will be explained. The applications include 2D MOFs as non-enzymatic electrochemical sensors, 2D MOFs as nucleic acid-based biosensors and 2D MOFs as electrochemical immunosensors.
Figure 8. The detection mechanism of 2D MOFs for various electrochemical biosensor applications.
Download figure:
Standard image High-resolution imageNon-enzymatic sensors
Due to the large surface area, good conductivity, fast electron and mass transfer of 2D MOF, this novel material becomes very potential in many electrochemical biosensor applications, specifically for non-enzymatic electrochemical sensors. Compared with the enzymatic electrochemical biosensors that commonly use 3D MOFs, the non-enzymatic electrochemical biosensor is more interesting to be explored because there are many advantages based on their stability in ambient conditions such as thermal and pH, easy to use, high sensitivity, good selectivity, and low cost.102 In recent years, some studies have reported the development of 2D MOFs as sensing material for non-enzymatic electrochemical biosensors. This mechanism is based on high electrocatalytic activity of 2D MOFs that can catalyze the reduction and oxidation reaction toward the analyte such as glucose, hydrogen peroxides, dopamine, ascorbic acid, and uric acid that can indicate the health condition of human bodies.
Besides resulting in a high sensitivity, the applied 2D MOFs as electrochemical non-enzymatic sensors also displayed excellent selectivity even in mixed compound-based detection. The selectivity generally is due to the size exclusion (molecular sieving) mechanism wherein atoms or molecules that are smaller than the MOF's apertures can be adsorbed, but larger molecules cannot.103 Designing pore and aperture size for MOFs is an essential consideration to determine the selectivity of the proposed MOFs. Pore modulation can be achieved by removing non-structural ligands or solvent guest molecules from framework nodes or replacing node-coordinated solvent molecules with larger or smaller ligands.104
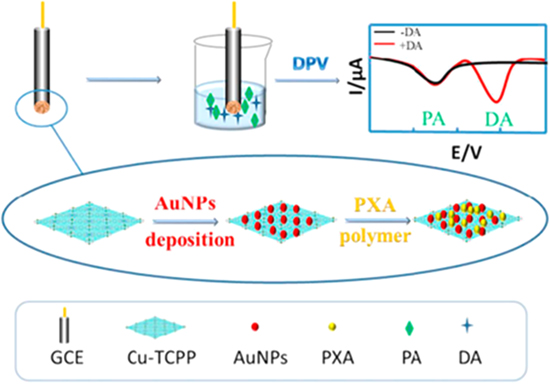
One of the analytes that can be detected using a non-enzymatic electrochemical sensor is dopamine. Dopamine, a type of neurotransmitter, is mostly found in the central nervous system and responsible for the hormonal response, cognition, and feelings in the human body.105 Low amount of dopamine may cause neurological disorders including Parkinson's, Huntington's, and schizophrenia.106 Qiu et al. proposed an electrochemical non-enzymatic biosensor for the detection of dopamine (DA) using 2D MOF nanosheets doped with Gold nanoparticles (AuNPs) and poly xanthurenic acid (PXA) composite. Their idea is to combine AuNPs with PXA and assemble it on the Cu-tetrakis (4-carboxyphenyl) porphyrin (TCPP) by using an electrodeposition technique to improve the electrocatalytic performance, as shown in Fig. 9. Under cyclic voltammetry measurement, the redox peaks current greatly increased when the (PXA) was incorporated into AuNPs/Cu-TCPP/GCE modified electrodes. This is due to the excellent electron transfer and good synergetic effect between AuNPs/Cu-TCPP/GCE and (PXA). Furthermore, in DPV measurement they used different concentrations of dopamine (DA) as the analyte and Paracetamol (PA) as the interference substance in an electrolyte solution since these analytes have nearly two different redox peaks that can be observed in a single measurement. The linearity and limit of detection (LOD) of the dopamine was 5–125 μM and 1.0 μM, respectively.95
Figure 9. The illustration of 2D Cu-TCPP doped with Gold nanoparticles (AuNPs) and poly xanthurenic acid (PXA) as non-enzymatic sensors for dopamine detection. Reprinted from publication95 with permission from Elsevier.
Download figure:
Standard image High-resolution imageAnother substance that is commonly analyzed by the electrochemical method is hydrogen peroxide (H2O2). H2O2 is naturally produced from an uncompleted metabolite of oxygen in living cells.107 However, an imbalance of H2O2 concentrations in the human body can indicate many diseases, such as cardiovascular disease, degenerative disease, or even cancer.108 The early detection of H2O2 is vital to prevent the malignancies of these diseases. Liu et al. synthesized two-dimensional NiCo-MOF nanosheets as non-enzymatic electrochemical biosensors for H2O2 detection. This 2D MOF nanosheets based on Co, Ni was synthesized at room temperature. By using cyclic voltammetry measurement, they obtained the redox peak potential of Co–MOF, Ni–MOF, and NiCo–MOF hydrothermal toward H2O2 was at 0.16 and 0.23 V, 0.25 and 0.5 V, 0.2 and 0.36 V, respectively. Besides, having the lowest working potential at oxidation potential of 0.25 V, Co–MOF displayed the best electrochemical response for H2O2 compared with other synthesized MOFs. The sensitivity of Co–MOF towards H2O2 was 0.69 μM with wide linearity of 0.5–832.5 μM.109
Similarly, Shu et al. reported non-enzymatic hydrogen peroxide detection using Ni–MOF and hemin. Hemin is a type of heme protein that has similar properties to the peroxidase enzyme. Hemin has several advantages compared to natural enzymes, including chemical and thermal stability, more resistant to denaturation, and low-cost synthesis process. To evaluate the electrochemical behavior of modified electrodes, the CV and DPV measurements were conducted. The combination of MOFs/Hemin/GCE, have the highest redox peak currents in hydrogen peroxide detection as compared to GCE/MOF or GCE/Hemin. This is due to the combination of the large surface area of Ni–MOF and the high peroxidase-like activity of hemin. These fabricated biosensors displayed a low detection limit of 0.2 μM with the linearity of 1 μM–0.4 mM towards hydrogen peroxide.110
Moreover, 2D MOFs which form nanosheets structure show more active sites, increased stability, and also more uniform dispersibility in solution, making this material also often used as an electrochemical biosensor material for glucose detection. The detection amount of glucose is crucial for diabetic patients. The quantitative detection of glucose can be used for monitoring the therapy, the severity of the disease, and determining the effectiveness of the drug delivery system. Li et al. reported the superior non-enzymatic glucose sensor using Co–MOF on a nickel foam array. The synthesis process used a hydrothermal method in aqueous solution. Even though bare nickel foam (NF) only provided the ability for glucose redox reaction due to the presence of nickel as the transition metal that can catalyze redox reaction, they claimed that there were quite weak responses towards glucose. Thus, the Co–MOF with excellent electrocatalytic performance is needed. After combining Co–MOF with NF, the electro-oxidation peak of glucose significantly increased. The measurement was taken in the alkaline condition which is at pH 13, the optimum value that was achieved-as we know that the electrochemical measurement of glucose can be easily conducted under base condition. However, if the measurement is established at pH over 13, this can cause an interference peak of the glucose oxidation since the oxygen evolution reaction phenomena occur at pH 14. Finally, based on their study, Co-MOF/NF exhibited strong activity for glucose detection in alkaline media. The detection of glucose was achieved with a wide linear range of 0.001 mM–3 mM and limit of detection was 1.3 nM.111
Xue et al. reported glucose detection using nickel modified copper metal organic framework (Ni@Cu-MOF) nanosheet. The synthesis process was prepared by a simple method at room temperature. Ni@Cu-MOF nanocomposite modified electrodes can be used for glucose detection without any further modification. The same as previously explained work, the electrochemical measurement establishes in alkaline conditions. The doping of Ni can enhance the conductivity and mobility of Cu–MOF modified electrodes. However, too much Ni accumulation can reduce these properties due to the covered surface of the electrode. This strategy displayed a wide linear range of glucose detection (5 μM to 2500 μM) with LOD of 1.67 μM.112
Furthermore, the promising thing is, if we were able to make 2D material in a vertical orientation. With this orientation, more available active areas can be exposed and consequently can provide a faster mass and electron transfer. Li et al. designed vertical two-dimensional NiCo–MOF nanosheets and demonstrated its application as a non-enzymatic glucose sensor.42 Usually, the synthesis of 2D MOF uses bottom-up strategy which needs a large amount concentration of surfactants113,114 and also required long synthesis process time.54 The most challenging thing is to prepare 2D MOF nanosheets with high uniformity without using any surfactant agents-considering that surfactants have several deficiencies. Therefore, establishing 2D MOF on conductive substrates which were proposed by Li et al. to be the solution of this problem. The good interactions between Nickel and Cobalt in the NiCo–MOFNs resulting in an excellent catalytic activity towards glucose. Under amperometric measurement, the linear range of glucose detection was from 0.0010 to 8 mM with a limit of detection of 0.29 μM.42
Nucleic acid biosensors
As we know, the large active surface area of 2D MOF can increase the loading efficiency and available site of biomolecules probing that is very needed in electrochemical biosensor applications, especially for nucleic acid-based biosensor.115 One type of nucleic acid biosensor that is commonly used is the aptamer biosensor (aptasensor). Aptamers is oligonucleotides that can be formed as signaling DNA (sDNA) or capturing DNA (cDNA) which commonly used for various target detection such as cancer makers, protein, or heavy metal ions.116 Aptasensor have many advantages compared with traditional nucleic acid recognition such as high stability, inherent selectivity and excellent bio-affinity. Recently, some studies have been reported for developing aptamers in biosensor application for measuring some tumor markers, which become a significant contribution in cancer monitoring, including evaluation of therapy and predicting cancer recurrence.117 In the biomolecules immobilization strategy to the 2D MOF surfaces, the majority of studies reported the absence of surface treatment for covalent linkages such as cross-linking agents since that 2D MOF nanosheet provide a large active site for π−π stacking, hydrogen bonding and electrostatic interactions for bioconjugation. Nevertheless, a small number of studies also reported the use of EDC/NHS as a cross-linking agent for covalent interactions. Cross-linking agents are chemical reagents that chemically activate a molecule, enabling it to directly couple with another molecule through covalent bonding. Immobilization chemistries using EDC/NHS cross-linker created an activated surface after forming an amide bond between the amine-functionalized substrate and the carboxyl group on the antibody.118 This strategy resulted in some advantages, such as not-requiring antibody modification, oriented antibody, prolonged stability, and commercial availability. However, the requirement of additional process steps and susceptible to hydrolysis still a limitation of this method.119
He et al. proposed 2D zirconium-based metal-organic framework (521-MOF) for the detection of mucin 1.117 Mucin 1 (MUC1) is a type of transmembrane protein that commonly presence at cancer malignancies such as breast cancer, ovarian cancer, prostate cancer, and pancreatic cancer.120 They synthesized 521-MOFs using polyvinyl pyrrolidone (PVP) as a capping agent to prevent the agglomeration, then followed by a synthesized processes under mild conditions to create a nanosheet structure. The immobilization of aptamer is achieved by strong bio-affinity between the 521-MOF and the aptamer strands without using any additional material like noble metal nanoparticles for bioconjugation or any surface treatment. The sensitivity of Mucin 1 detection was evaluated by the EIS method with a linear range from 0.001 to 0.5 ng·ml−1 and low detection limit of 0.12 pg·ml−1.117
In recent years, bimetallic MOFs have become an exciting topic for MOFs study due to the unique combination characteristic between two metals in one structure. Some studies showed that bimetallic MOFs have more hydro stability compared with single metal MOFs.46 Based on this idea, Zhou et al. synthesized two-dimensional ZnZr bimetallic MOF for cancer marker protein tyrosine kinase-7 (PTK7) detection.44 Bimetallic ZnZr–MOF was obtained by changing the synthesis parameters such as the sequence of metal ion precursors and organic ligands to get Zn-MOF-on-Zr-MOF. As we know the organic ligands in MOFs commonly have specific functional groups that can provide an available sites for immobilization of biomolecules via π−π interactions, covalent attachment, hydrogen bonding, or electrostatic interactions.121 Since the high affinity of biomolecule binding from Zr-based MOF, the immobilization of the aptamer onto the Zn-MOF-on-Zr-MOF can be achieved. To evaluate the electrochemical characteristics of the developed aptasensors, the EIS method was used. After aptamer immobilization, the impedance of modified electrodes was significantly increased due to the limitation of the access of redox ion mobility to the electrode surface. In the presence of PTK7, the diameter of the Nyquist plot increased due to the G-quadruplex interaction between aptamer and PTK7. To evaluate the sensitivity performance, the EIS method was used again which results in the limit of detection (LOD) on PTK7 was 0.84 pg ml−1 within the concentration range of 0.001–1.0 ng ml−1.44
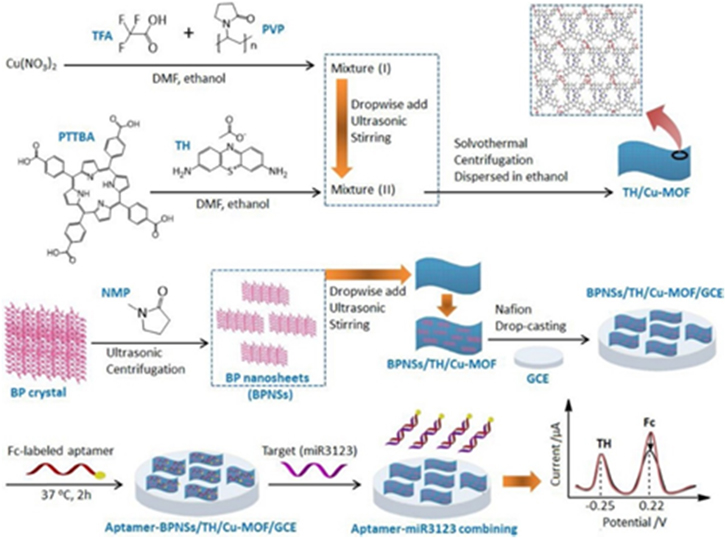
Meanwhile, the quantification of MicroRNA (miRNAs) also plays an important role in many clinical diagnoses of dangerous diseases, including cancers, heart diseases, neurological diseases, and many more. Sun et al. developed Black Phosphorus nanosheet and metal organic framework composite (BPNSs/MOF) for accurate detection of miR3123 as a biomarker for gastric cancer. They prepared 2D MOF via the solvothermal method, using Cu(II) as metal ion and PTTBA as organic ligand, as shown in Fig. 10. On the other hand, thionine (TH) was used as doping material and it was combined with BPNSs to immobilize the aptamer. Moreover, the successful synthesis process of modified electrodes was evaluated by CV and EIS methods. The performance detection of miR3123 was obtained by plotting linearity between the peak current of ferrocene (IFc) and the peak current of thionine (ITH). The ratio of IFc/ITH is known as ratiometric electrochemical. Due to the increasing of CmiR3123 concentrations, IFc regularly reduced and ITH hardly changed. This strategy resulting in a linear the range of miR3123 from 2 pM to 2 μM and limit of detection (LOD) of 0.3 pM.122
Figure 10. The synthesis process of the nucleic acid-based biosensor for the detection of miR3123 and the sensing mechanism using BPNSs/MOF. Reprinted from publication122 with permission from Elsevier.
Download figure:
Standard image High-resolution imageBesides the strategies for developing MOF on MOF 2D materials as mentioned in the previous paragraphs, the growth 2D MOF on COF (Covalent organic frameworks) is also a fascinating topic. Due to the combination between sensitivity and selectivity of aptasensors, easy operation of electrochemical techniques, as well as excellent electrochemical activity and strong bio-affinity towards biomolecules, makes it very interesting to be explored as novel biosensors.123 Liu et al. designed MOF on COF composites based Co–MOF and TPN–COF as label-free aptasensor for ampicillin (AMP) detection. AMP is a contaminant which is often found in agricultural products and waters that can have an impact on allergic reactions, difficulty breathing, and spasms in the human body The aptamer immobilization can be obtained through a very strong bio-affinity between the aptamer strand and the Co-MOF@TPN-COF matrix via π-π stacking and hydrogen bonding. The electrochemical characteristic for this developed aptasensor was evaluated using the EIS method. The limit of AMP detection reached 0.217 fg ml−1 with the linearity from 1.0 fg ml−1 to 2.0 ng ml−1.124
By using the electrochemical luminescent technique, demonstrated by Shao et al., synthesized Ru–MOF nanosheet for labeled miRNA-141 detection. In their study, Ruthenium-based MOF was used as a signaling unit because of the excellent catalytic activity of Ru–MOF nanosheet. The immobilization of labeled signaling of DNA (sDNA) on the Ru–MOF surface was achieved by zero-length crosslinking agent for activated carboxylate group on Ru–MOF and forming an amide bond with sDNA. Meanwhile, the capturing DNA (cDNA) was attached on Fe3O4@SiO2@Au composite surface that has a function as a capturing unit, shown in Figs. 11a–11c. After that, cDNA will hybridize with miRNA-141 target following with the dropping of Ru-MOF labeled sDNA as a signal unit above it. This resulted in a significant enhancement of sensing performance and sensitivity of the sensor. This strategy achieved a wide linear range for miRNA-141 detection at 1 fM–10 pM with LOD of 0.3 fM.123
Figure 11. (a) The fabrication process of capturing units based on magnetic nanoparticles, (b) the fabrication process of signaling units based on Ru-MOF labeled sDNA, and (c) the final electrode preparation for analyte measurement. Reprinted from publication124 with permission from Elsevier.
Download figure:
Standard image High-resolution imageImmunosensors
Immunosensors based on antigen and antibody reactions can be considered as clinical analysis for sensitive detection of disease-related proteins.125 Compared with conventional enzyme-linked immunosorbent assays (ELISAs), electrochemical immunoassays have much advantages, such as high sensitivity, high selectivity, low cost, easy to use, portability and fast analysis.126 Dong et al. reported the detection of calprotectin (CALP) by using two-dimensional Cu-TCPP(Fe) doped PtNi nanosheet.127 CALP is a biomarker for several melioidosis diseases that can be used to evaluate treatment responses to antibiotics.128 CALP monitoring in patients is vital to evaluate the severity and effectiveness therapy of inflammatory diseases in clinical diagnosis. On the other hand, the bimetallic Cu-TCPP(Fe) nanosheets with their large active surface area permit more binding sites for PtNi attachment, resulting in the increasing electrochemical catalytic activity and also provide more active sites for antibody immobilization. Based on CV curve and the EIS plot, they claimed successful electrode preparation including immobilization of antibodies and the detection of CALP. This labeled immunosensors resulting in a low detection limit of 137.7 fg ml−1 within the range of 200 fg.ml−1 to 50 ng.ml−1 towards CALP.127
Similarly, Xiao et al. developed two-dimensional Cu-TCPP(Fe) and polyethyleneimine (PEI) composite for sulfonamide detection.129 Sulfonamides (SAs) is generic level antibiotics that can become contaminants in the aquatic environment through feces and urine released by patients that can contribute to the evolution of antibiotic-resistant genes in the environment and potential damage to human health.130 Interestingly, the Cu-TCPP(Fe) structure is destroyed when the introduction of polyethyleneimine (PEI) from PEI graphene and SAs antibody composite (PEI-GO/Ab) by the stronger affinity interaction between PEI and Cu2+ than that of Cu-TCPP(Fe). These phenomena result in a decreased electrochemical signal of immunosensor and can be used for SAs antibody detection. The electrochemical properties of the fabricated immunosensors were analyzed by EIS method under the optimum conditions. This strategy had a linear range of 1.186–28.051 ng ml−1, with a low detection limit of 0.395 ng ml−1.129
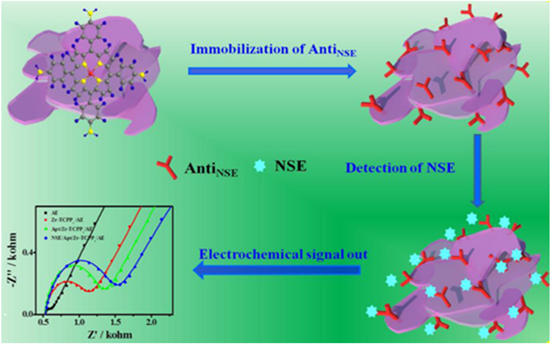
In a different point of view, Li et al. proposed Zirconium-porphyrin composite as label-free immunosensors for enolase detection.131 This complex Zr-TAPP was established from Zr(III) as metal ion and tetra aminophenyl porphyrin (TAPP) as organic ligand. The neurons specific enolase (NSE) is the type of YY dimer enzyme that is widely distributed in mammalian tissues.132 NSE can be used as a biomarker for various diseases related to the nervous system such as Alzheimer's, neuroblastoma, and even neuroendocrine diseases because there will be an increase in NSE levels when a person is diagnosed by this disease.133 Several advantages is possessed by the Zr-TAPP complex, including abundant N-related functional groups, high π-π stacking, and high hydrophobicity. These advantages not only can increase the antibody immobilization capacity which will increase efficiency in capturing antigens, but also can increase the stability of complex interactions between antibodies antigen in aqueous solution, as shown in Fig. 12. Under EIS measurement for NSE detection, the Zr-TAPP-based immunosensor showed a low detection limit of 7.1 fg ml−1 with a linear range of 10.0 fg ml−1 to 2.0 ng ml−1.131
Figure 12. Illustration of label-free immunosensor mechanism for NSE detection. Reprinted from publication131 with permission from Elsevier.
Download figure:
Standard image High-resolution imageOn the other hand, combining electrochemical and luminescent techniques, i.e. electrochemiluminescent (ECL), is also an auspicious thing, which provides many advantages in an immunoassay such as wide linear range, dissolving low background signal problem, accessible to modified and adjustable performance. Generally, ECL technique uses luminol and noble metal nanoparticles (MNPs) composite as sensing platforms for many applications. Unfortunately, the classical problem in noble metal synthesis is the agglomeration process that can affect the immunosensor performance. Therefore, Wang et al. proposed a metal-organic framework with high porosity and large surface area for luminol-MNPs platform to prevent agglomeration phenomena of MNPs. The combination between Co/Ni MOF and luminol-AgNPs was used for the detection of alpha-fetoprotein (AFP). Low concentration of AFP in the human body is responsible for many liver cancer cases, so that early detection of AFP is very crucial, especially for humans that are infected by hepatitis B or Hepatitis C. This developed immunosensor resulting linear range from 1 pg ml−1 to 100 ng ml−1 with LOD of 0.417 pg ml−1 towards AFP.134
Lastly, the summary of 2D MOFs applications as electrochemical biosensors are shown in Table III.
Table III. Electrochemical biosensor performances based on 2D MOFs.
| 2D MOF Modified Electrode | Method | Analyte | Linear Range | LOD | References |
|---|---|---|---|---|---|
| 2D MOFs as Non-enzymatic electrochemical sensors | |||||
| NiCo-MOFNs | Amperometric | Glucose | 1 μM–8 mM | 0.29 μM | 42 |
| Co-MOF/GCE | Amperometric | H202 | 0.5–832.5 μM | 0.69 μM | 109 |
| Cu-TCPP/Au/p(XA)/GCE | DPV | Dopamine | 5–125 μM | 1.0 μM | 95 |
| Ni-MOF/Hemin/GCE | DPV | H2O2 | 1 μM–0.4 mM | 0.2 μM | 110 |
| Co-MOF/NF | Amperometric | Glucose | 0.001 mM– 3 mM | 1.3 nM | 111 |
| Ni@Cu-MOF/GCE | CV | Glucose | 5 μM–2500 μM | 1.67 μM | 112 |
| 2D MOFs as electrochemical Nucleic Acid based Biosensors | |||||
| Apt/521-MOF/AE | EIS | Mucin 1 | 0.001–0.5 ng ml−1 | 0.12 pg ml−1 | 117 |
| SGC8/Zn-MOF-on-Zr-MOF/AE | EIS | PTK 7 | 0.001–1.0 ng ml−1 | 0.84 pg ml−1 |
|
| aptamer-BPNSs/TH/Cu-MOF/GCE | SWV | miR3123 | 2 pM–2 μM | 0.3 pM | 122 |
| Apt/Co-MOF@TPN-COF/AE | EIS | AMP | 1.0 fg ml−1–2.0 ng ml−1 | 0.217 fg ml−1 | 124 |
| Ru-MOF/Fe3O4@SiO2@Au | ECL | miRNA-141 | 1 fM–10 pM | 0.3 fM | 123 |
| 2D MOFs as electrochemical Immunosensors | |||||
| PtNi@Cu-TCPP(Fe)-Ab2/BSA/Ab1/Au@MWCNTs/GCE | Amperometric | CALP | 200 fg ml−1–50 ng ml−1 | 137.7 fg ml−1 | 127 |
| PEI-GO@Ab2/Ab1/BSA/Ag/Cu-TCPP(Fe)/MWCNTs/GCE | Amperometric | SMM | 1.186–28.051 ng ml−1 | 0.395 ng ml−1 | 129 |
| AntiSNE/Zr-TAPP/AE | EIS | NSE | 10.0 fg ml−1–2.0 ng ml−1 | 7.1 fg ml−1 | 131 |
| Co/Ni MOF/luminol-AgNps | ECL | AFP | 1 pg ml−1–100 ng ml−1 | 0.417 pg ml−1 | 134 |
Conclusions
In this review, we comprehensively discuss the 2D MOFs in electrochemical biosensor applications. We introduce the structures and synthesis method of 2D MOFs and their applicability as non-enzymatic sensors, nucleic acid biosensors, and immunosensors. We also discuss their performance and also the corresponding limit of detection as an electrochemical biosensor.
The studies on complex nanostructures of MOFs have now been massively studied and have benefited in several sectors. One of them is the fundamental relationship between complex nanostructures and their electrochemical properties. On the other hand, theoretical calculations and modeling are still very rarely studied. However, it can provide several new opportunities to be developed.135 Even though there are some advantages in the form of porous structure and chemical composition that are easily controlled, it should be noted that the use of MOFs in research is still in its early stages and still faces some challenges.18 Therefore, further development of MOFs is required; one of them is made into a two-dimensional. As future perspectives, 2D MOFs are great opportunities to answer the challenges of electrochemical biosensors because of their unique properties. They can give better efficiency than 1D and bulk MOFs. This way will make MOFs have more flexibility, increase the density of active sites, produce hollow or porous structures, and also the possibility to build hybrid architectures.136 based on these facts, 2D MOFs as electrochemical biosensors can be applied to many fields such as nanoscience and biotechnology. With the development of various fields, such as nanoscience and biotechnology, MOF-based materials will demonstrate up-and-coming applications and offer a proof-of-concept as/for electrochemical biosensor.137 Various electrochemical biosensors utilizing a combination of nanotechnology and 2D MOFs have been demonstrated can improve the performance with rapid, accurate, and sensitive biological sample detection. Detailed comparative analysis between various nanostructured materials has confirmed that the sensor's sensitivity can be significantly increased through various routes (e.g., chemical functionalization, synthesis of hybrid composites, and modulation of the surface architecture of these nanomaterials).138 With the joint efforts of experts from the multidisciplinary fields, one can expect that many breakthroughs will be achieved in the near future.139
Challenges/Future Works/Opportunities
2D MOF materials have been demonstrated as innovative sensing platforms for electrochemical sensing applications. This review overviews recent developments in 2D MOF materials in this emerging field with particular interests about the synthesis–property-application relationship and different strategies for improving the sensitivity, selectivity, stability, and reliability of the 2D MOF-based electrochemical sensors. To obtain an electrochemical sensor with the desired sensitivity, developing nanoscale MOFs with unique structures such as 2D MOF nanosheets to improve the electroactive area of the electrode, and promote the mass/electron transfer efficiency will be an effective method. 2D MOFs composited with conductive functional species, such as carbon nanomaterials or metal nanoparticles to promote the uniform distribution and exposition of more active sites, will be another practical approach to obtain high sensitivity. Moreover, the composition of 2D-MOFs with biomolecules, such as enzymes, antibodies, and aptamers with specific recognition functions, will be the most effective method to improve the sensor's selectivity. Developing ratiometric electrochemical sensors by introducing a reference signal will also help achieve reliable and accurate sensors. To design MOF-based electrochemical sensors for particular analytes, careful consideration of all these aspects mentioned above to optimize the sensing performance will be necessary. Despite the significant progress, the practical application of 2D MOF-based electrochemical sensors is still in its early stage. Many challenges remain to be resolved for advancing the application of MOF-based materials in electrochemical sensors.
In general, the mechanisms through which 2D MOF-based materials sense different analytes are still poorly understood. It is generally believed that the electroactive MOFs or MOF derivatives serve as the sensing sites and behave like the electrocatalyst to catalyze the redox reaction of specific analytes, thus giving the redox current signals. For MOF/metal nanoparticles and MOF/enzyme-based electrochemical sensors, the electroactive metal nanoparticles or enzymes serve as the sensing sites and catalyst to give the redox current signals. At the same time, MOFs provide a framework with high surface area, high selectivity, and high diffusion. For MOF/antibody and MOF/aptamer-based electrochemical sensors, the antibodies or aptamers serve as the sensing sites and specific recognition with the analytes.
In contrast, MOFs with large specific surface areas and porous structures serve as the matrices to immobilize the biomolecules. For in-depth analysis and detection of the interactions between the sensing sites and the analytes, the application of new technologies, such as liquid HRTEM, liquid-phase AFM, for the direct and in situ observation of the guest-responsive behavior occurring on the surface of sensing sites during the sensing process will be beneficial. Additionally, continuing the study of the synthesis–property-application relationship of MOF-based materials for electrochemical sensing and developing new synthesis strategies to prepare materials with desired qualities, will undoubtedly have important theoretical significance and practical value for promoting the rapid development of this exciting field.
To be specific, for pristine MOFs, MOFs' inherent growth mechanism, especially MOFs grown on electrode surfaces, is still poorly understood at present. Further deepening the understanding and precise control over MOFs' assembly is of great significance for the rational design and controllable synthesis of MOFs with desirable properties for electrochemical sensing applications. Theoretical research using density functional theory (DFT) based on designed experiments will help reveal the exact growth mechanism of MOFs. Besides the stability, most MOFs' electrical conductivity and electrocatalytic activity must be further improved for electrochemical sensing applications. At present, only limited pristine MOFs, such as Cu-MOFs and Zr-MOFs, which feature both high stability and abundant active sites, can satisfy the various requirements of electrochemical sensing applications. In addition to the typical morphologies (e.g., polyhedra, rod shapes, or nanosheets), new topology structures, such as the MOF-based 1D nanofibers prepared via electrospinning, hollow MOFs, composites or derivatives, will be of great interest. For MOF composites, accurately controlling the assembly of MOFs and functional species at the molecular level would be of vital importance to deeply understand the synergy resulting from generating composites. Precisely positioning the guests, such as the aptamers in MOFs or the MOFs on graphene matrices or guest metal NPs relative to the host MOF particles, is still a significant challenge. Also, developing new nanomaterials, such as covalent organic frameworks (COFs), metal-organic gels (MOGs), and supramolecular hydrogels (SMGs), as appropriate matrices for achieving the excellent dispersion of MOFs is highly attractive but remains challenging. For MOF derivatives, achieving the scaled-up preparation of MOF-derived materials with high yields and low prices is still challenging. Additionally, an understanding of the structure and nanostructure evolution mechanism and the ability to control the MOF-derived materials during the conversion process remain unattained, severely restricting the rational design and functional applications of MOF-derived materials. In situ experiments and advanced characterization techniques will help clarify the transformation process and evolution mechanism.
Despite these challenges and due to the unique structural advantages of MOFs, we firmly believe that MOF-based materials have unique advantages and are promising for electrochemical sensing applications possibly in, but not limited to, the following fields: 1) Multiplex detection: in terms of practical applications, it is highly demanding to develop simple multiple analyte detection assays. However, the applications of MOF-based electrochemical sensors for multiplex detection, especially multiplex antibiotics or cancer marker detection, is still a new area to be explored. 2) On-site detection: at present, a MOF-based electrochemical sensing study is still being developed in a laboratory; by developing MOF-based electrochemical sensors with a screen-printing technique and 3D printed microfluidic devices, it is possible to realize real-time and on-site detection. 3) In vivo detection: at present, the research on MOF-based electrochemical biosensors has mostly been emphasized on in vitro detection only. The in vivo detection of biological molecules, biomarkers, pathogens, DNA, and others. is highly desirable for practical clinical diagnosis and treatments. The preparation of low-toxicity, biocompatible and biodegradable MOFs, such as cyclodextrin-based carbohydrate MOFs (CD-MOFs), uses endogenous organic linkers or bioactive substances as surface modifiers may be the first step to realizing the in vivo applications.
In general, the application of MOF-based materials in the electrochemical sensing field is still at an early stage, and there is plenty of room for innovative research in this exciting field. The rational design and controlled synthesis of multifunctional MOF-based materials for combined applications, such as biosensing, imaging, and controllable drug release, are highly desirable for the visualization and quantification of clinical diagnostics and therapeutics.
Acknowledgments
The authors acknowledge financial grants provided by Lembaga Pengelola Dana Pendidikan (LPDP), Ministry of Finance of Indonesia. This work is also partially supported by the Indonesia Ministry of Education and Culture, and the Indonesia Ministry of Research and Technology under the grant scheme of World Class University Program managed by Institut Teknologi Bandung. The authors also acknowledge the partial financial support fromDexa Science Scholarship.