Abstract
Low-cost and high-performance lithium ion batteries (LIBs) are a key technology in these days. One promising candidate for cathodes is the layered nickel (Ni)-rich LiNi0.8Co0.1Mn0.1O2 (NCM811) active material due to its high energy density, high specific capacity and lower material costs as well as social aspects concerning mining due to the diminished cobalt content. However, the lower thermal stability and higher sensitivity to H2O and CO2 result in a potential stronger performance degradation and lower safety. Therefore, process adaptions are inevitable. In this paper the current status and challenges of the entire cathode production process with NCM811 as active material are reviewed taking quality, cost and environmental aspects into account. General important aspects within the process are presented which are specially extended to NCM811 cathode production. Process recommendations are highlighted and innovative approaches like a water-based or solvent-free processing are discussed in comparison to conventional production technologies.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
The current efforts to decrease CO2 and NOx emissions encourage the importance of electrochemical storage technologies. 1 One key technology is the manufacturing of high-performance and low-cost batteries. In this context, lithium ion batteries (LIBs) consisting of a cathode, an anode, a separator and an electrolyte have come into focus. Promising active material candidates for cathodes are Ni-rich layered LiNixCoyMnzO2 (NCM) like LiNi0.8Co0.1Mn0.1O2 (NCM811). Latter one is characterized by a high energy density, high specific capacity and lower material costs. Table I demonstrates the high initial discharge capacity of cells with pristine NCM811 (not coated, no gradient or core–shell structure etc.) as active material measured by afferent labs. 2–5 In comparison to NCM materials with a Ni-content of ≤0.6 which are well investigated and commercialized Ni-rich materials are associated with a potential stronger performance degradation and lower safety. 6 Figure 1 illustrates the correlation between a high Ni-content and a high discharge capacity but low thermal stability and capacity retention. The next level in decreasing the Co-content are NCM materials with an Ni-content of ≥0.9 like LiNi0.9Co0.05Mn0.05O2. 7 Furthermore, Manganese (Mn)-rich NCM materials or phospho-olivines LiFeMnPO4 are examples for potential future active materials. 8,9 In the past years, electrode production technologies for high-performance lithium ion batteries like the mixing step of the raw materials or calendering procedure have been under constant optimization. 10–14 Although several studies concerning design strategies to enhance the stability of NCM811 and some studies about individual process steps like dispersion and cell behavior exist, there is no comprehensive information about the entire cathode production with a Ni-rich active material like NCM811 yet. This article aims to review the current status and challenges of the promising active material with respect to the entire cathode production from the formulation design to post-drying procedure. The most important design goals herein are a high energy and power density, high cycling stability, good safety, low costs and social aspects. 11,15 Due to the higher sensibility of the Ni-rich material to H2O and CO2 one of the most important aspects is the provision of an adequate process environment.
Table I. Discharge capacity of cells with pristine NCM811.
| Voltage Range [V] | C-rate [C] | Discharge Capacity [mAh g−1] | Temperature [°C] | Cell Type | References |
|---|---|---|---|---|---|
| 3.0−4.3 | 0.1 | 198 | 30 | Coin | 4 |
| 3.0−4.4 | 0.2 | 197 | 30 | Coin | 2 |
| 2.8−4.3 | 0.1 | 201 | 25 | Coin | 3 |
| 2.5−4.4 | 1.0 | 185 | 25 | Pouch-type graphite full cell | 5 |
Figure 1. Characteristics of NCM active materials with different stoichiometric compositions. In the colored oval the blue point demonstrates the thermal stability corresponding to the left axis and the red point marks the capacity retention corresponding to the right axis. Reprinted from Ref. 16. Copyright 2013, with permission from Elsevier.
Download figure:
Standard image High-resolution imageChallenges of Ni-rich NCM811
Understanding the properties of the active material and its challenges is inevitable to create an efficient cathode production chain. One of the main problems is the oxidation of lattice oxygen and the release of singlet oxygen occurring at approximately 80% state-of-charge and high degree of delithiation.
17
According to this, a decline of the onset potential of oxygen evolution from approximately 4.7 V vs Li/Li+ for NCM111 and NCM622 to 4.3 V vs Li/Li+ for NCM811 is described. Furthermore, the CO and CO2 release correlates with the oxygen evolution due to a reaction between the singlet oxygen and electrolyte solvent. Probably, the released lattice oxygen reacts with ethylene carbonates under the formation of CO2, CO and H2O.
18
It is expected that the oxygen release is also strongly connected with the formation of a disordered spinel and/or rock-salt phase on the NCM particle surface.
17
Another study confirms a transition from a layered R  m to a Fm
m to a Fm  m rock-salt structure during cycling and electrolyte exposure.
19
The resulting rock-salt NiO phase is associated with a higher impedance of the battery.
6
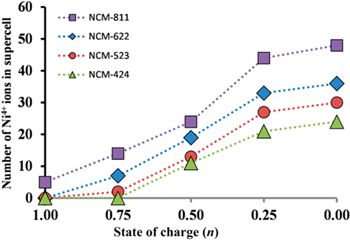
Furthermore, the amount of high-valence Ni4+ ions increase with ascending Ni-content of the NCM and increasing state of charge resulting in covalent Ni–O bonds and undesirable side reactions (Fig. 2).
20
m rock-salt structure during cycling and electrolyte exposure.
19
The resulting rock-salt NiO phase is associated with a higher impedance of the battery.
6
Furthermore, the amount of high-valence Ni4+ ions increase with ascending Ni-content of the NCM and increasing state of charge resulting in covalent Ni–O bonds and undesirable side reactions (Fig. 2).
20
Figure 2. Amount of Ni4+-ions in various NCM materials in dependence to state of charge. These are theoretical results estimated by computed magnetic moments and the projected density of states. Reprinted with permission from Ref. 20. Copyright 2017, American Chemical Society.
Download figure:
Standard image High-resolution imageAnother frequently reported problem of NCM material is cation mixing, which describes the occupation of the Li+ ion positions within the lattice by other metal ions. Because of the similar atomic radii, the mixing mainly occurs between Ni2+ and Li+ ions with a radius of 0.69 Å and 0.76 Å, respectively. 6,21,22 Consequently, the problem increases with ascending Ni-content. As a result of the mixing, the diffusion paths of the Li+ ions are blocked and the ion's mobility is diminished. 6 Besides, it is assumed that microcracks within the active material result from a decreasing unit cell volume at high degree of delithiation (Fig. 3a). 23,24 The unit cell volume is mainly controlled by the lattice c-axis which decreased abruptly at a lithium content of ≤0.5 (Figs. 3b and 3c). X-ray diffraction measurements revealed an anisotropic shrinkage of the unit cell volume from 101.4 Å3 at a lithium content of 1 to 94.3 Å3 at a lithium content of 0.25 (Fig. 3d). 24 It is assumed that the shrinkage of the layered oxides is predicated on covalent MO–OM peroxide bonds formed by the oxidation of lattice oxygen. 6 Consequently, the electrolyte invades into the cracks and is decomposed. Also, it initiates a dissolution of the transition metals within the active material. 23,25
Figure 3. (a) Microcracking and electrolyte invasion within NCM active material with different stoichiometric composition. Reprinted with permission from Ref. 25. Copyright 2018, American Chemical Society. (b) Crystal structure of NCM811. Transition-metal layers are shown in gray and lithium interlayers in green. Transition-metal-oxygen (hTM-O) and lithium-oxygen (hLi-O) slab heights are illustrated as well as the orientation of the lattice parameters a and c. Changes in the (c) lattice parameters a and c and (d) unit cell volume as a function of lithium content obtained by XRD on a Li/NCM811 cell. Reprinted with permission from Ref. 24. Copyright 2017, American Chemical Society.
Download figure:
Standard image High-resolution imageAtmospheric Impact
Several studies report a detrimental impact of H2O and CO2 from the atmosphere on the NCM active material by parasitic side reactions. 6,26–28 Therefore, one processing approach of major importance is the regulation and controlling of atmospheric parameters. One arising problem in the ambient environment is the formation of residual lithium compounds (RCLs) with strong alkalinity on the material's surface. It is suggested that a reduction of Ni3+ to Ni2+ can lead to a formation of O2− on the surface of the NCM particles. Possibly, it reacts with H2O and CO2 from the atmosphere and Li+ under the formation of the RLCs LiOH and Li2CO3 according to the following Eqs. 1 and 2 6,29 :
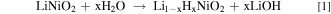
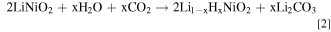
However, it is well known that the amount of the RLCs increases with an ascending Ni-content and humidity. 16,27,30 Li2CO3 is believed to be responsible for battery swelling especially during storage at high temperatures in the charged state which increases the risk of fire. The reaction of LiOH with polyvinylidene difluoride (PVDF) based binders could lead to a gelation of the cathode slurry during the coating process. 6,31 Furthermore, it is believed that a delithiation layer is formed near the surface region as a consequence of the RCLs. 26 Apart from this, RCLs can also be generated as a result of an excess LiOH supply during material synthesis to compensate for a loss of Li2O. 6 Further findings suggest a formation of transition metal hydroxides and carbonates on the surface of pristine NCM material in the presence of moisture and CO2 according to Eqs. 3 and 4 27 :
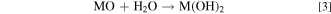
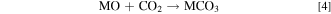
In this context, it is assumed that the main carbonate surface contamination on Ni-rich NCM811 is nickel carbonate which could also contribute to a gassing behavior. 27 Furthermore, an amount of > 150 μmol gNCM −1 hydroxides and carbonates of a liquid NCM622 cathode suspension already leads to a flocculation and gelation. 28,32 In addition, a consequence of residual water content within the battery is the formation of gaseous hydrogen fluoride (HF) due to a reaction with the commonly used conductive salt LiPF6 33 :
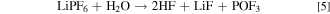
This reduction leads to a loss of the conductive salt and hence, a deteriorating cell performance due to a higher electrical resistance. 34 Furthermore, HF induces a decomposition of transition metals resulting in (fluoro)phosphates, CO2, oligocarbonate based products, diols and alkyl fluorides. 35 As a result of the acidic products, the solid electrolyte interface (SEI) cannot be formed or is attacked. 33 Findings also suggest that cathodic-site water can be distributed throughout the entire battery cell and hence, affect the anode. However, a small water content could favor the formation of a SEI on the anode. 34 Nevertheless, humidity should be kept to a minimum during the material production, storage and entire NCM811 cathode manufacture as well as subsequent cell production. This requires a dry and inert atmosphere with a low dew point. Whether a CO2 free atmosphere can result in a further reduction of surface contaminations should be investigated. Possibly, a water content under a critical value can already be sufficient to interrupt parasitic side reactions. However, specially designed dry rooms with an adjusted atmosphere can provide an effective processing for Ni-rich cathodes and also deliver a CO2 controlled atmosphere.
Design Principles for NCM811 Particles
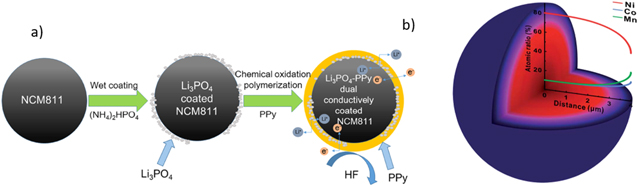
Different design strategies for the NCM811 particles are applicable to enhance the electrochemical performance of the cathode. A surface coating is described as an effective method to reduce side effects between the layered oxides and the electrolyte or the atmosphere, respectively. For example, a Li2SiO3 coating or a dual conductive layer composed of Li3PO4 and polypyrrole (PPy) are described for NCM811 (Fig. 4a). 36,37 In addition, some coated layers can provide doping ions to reduce the cation mixing. In this context, a retention capacity of 76% after 170 cycles at 1 C between 2.7 V and 4.3 V at 25 °C is described for NCM811 coated particles with Li4Ti15O12 compared to 39% for bare NCM particles. 38 Other strategies described in the literature to reduce RLCs are providing an excess of lithium sources or an enhancement of the partial pressure of oxygen. 6,39 Furthermore, a gradient nanostructure of the active material can improve the cathode's performance. Here, the Ni-content decreases from the core to the surface of the particle. For example, a gradient structure is described with NCM811 at the core decreasing to NCM523 at the surface achieving a discharge capacity of 190 mAh g−1 at 3.0 V to 4.0 V and a capacity retention of 86.5% after 300 cycles at 1 C (Fig. 4b). 40
Figure 4. Design strategies. (a) Li3PO4-PPy dual-conductively coated NCM811 particle. Reprinted with permission from Ref. 37. Copyright 2017, American Chemical Society. (b) Gradient structure of a NCM particle with decreasing Ni-content to the surface. Republished with permission of The Royal Society of Chemistry from Ref. 40. Copyright 2019, permission conveyed through Copyright Clearance Center, Inc.
Download figure:
Standard image High-resolution imageAnother strategy to enhance material stability uses a core–shell-structure of the NCM811 particles. 41,42 One study describes a core of NCM811 and a shell of Li2MnO3. Here, it is reported that cells with the core–shell material can be cycled to a cut-off voltage of 4.7 V in contrast to 4.3 V for the pristine material. 42 Another study indicates a reaction between dimethylglyoxime and Ni-ions to form a Ni-rich core and a Mn-rich shell. 41 This way the Ni-rich core can be protected from harmful reactions with the atmosphere or electrolyte solution. Another core–shell concept is to coat NCM111 onto NCM811 during a co-precipitation to receive an electrochemically active surface. However, the pristine material shows a higher initial capacity but poorer capacity retention especially at higher temperature: For the pristine material an initial discharge capacity is described of 180 mAh g−1 and a capacity retention of 74% and for the coated material an initial discharge capacity of 169 mAh g−1 and retention of 90% after 100 cycles at 60 °C (2 C, 2.8–4.3 V). 43 A consideration to reduce the formation of metal carbonates in ambient atmosphere is to inertize the metal oxide based active material. 27 Cation mixing can be reduced by doping with foreign ions into the Li or Ni sites. Improved cycle stability is already confirmed for NCM811 cathodes by this method. 44,45 In this respect, it is recommended to use cations with electronic noble gas configuration (e.g. Al3+, Mg2+) or cations with a higher potential compared to the operating potential of the cathode (e.g. Zr4+, Ti4+). 6 Concerning the prevention of microcracks, it is suggested that a radial crystallographic texture of NCM particles with a Ni-content of x ≥ 0.8 could diminish a propagation of microcracks through the surface. This way, a random expansion and contraction of the primary particles is avoided. 25 In summary, an improved material stability due to a surface coating, doping with foreign ions or a core–shell/gradient structure of the NCM811 particles leads to a better cell performance. For further reading concerning the problems of Ni-rich material and possible material based solutions different reviews 6,46–48 describing the subject in more detail are recommended.
Electrode Production
Generalized electrode production chain
Electrode production technologies have been under constant optimization in the past years. 10–14,32,49–51 To get a basic understand a generalized production chain is described in this section, as it can be performed for NCM811 containing cathodes (Fig. 5). Subsequently, the following sections go into more detail about the processing of NCM811. Conventionally, cathode production is based on wet processes. 11 Olivines (e. g. LiFePO4), layered oxides (e. g. LiNiCoMnO2, LiNiCoAlO2) and spinels (LiMnO4) are used as active material. Before being coated onto the current collector, which is usually aluminum, the active material is mixed with conductive additives (e. g. carbon black, carbon fibers, conductive graphite) and polymer binder. For cathodes, in most cases polyvinylidene difluoride (PVDF) serves as a binder, which requires the use of N-methyl-2-pyrrolidone (NMP) as solvent. 11,52
Figure 5. Generalized electrode production chain.
Download figure:
Standard image High-resolution imageInitially, a conditioning of the selected raw NCM material can be conducted like a washing procedure to reduce surface contaminations. 53–57 Especially for the highly sensitive NCM811 it is important to minimize parasitic surface contaminations. Usually, prior to the wet dispersing materials are mixed in dry mode for homogenization and sometimes first carbon black deagglomeration. 11 The deagglomeration degree of carbon black can be influenced by mixing time and intensity as well as by a proper selection of the carbon black type, which can differ in primary particle size and hence show a different breaking behavior. 10 For the dispersing step, the active material, conductivity additives and binder are fed into the solvent in a planetary mixer or extruder. The binder can be added to the dry mixing process or dissolved in the solvent before the conductive additives are dispersed. 11 A solids content between 70% and 75% is recommended as a compromise between good electrochemical performance and costs for the manufacturing of NCM cathodes by a continuous extruder-based process. 14 After dispersing, the suspension is degassed and filtered to receive a homogeneous suspension. Important suspension properties herein are the particle/agglomerat size distribution of carbon black. At industrial scale, the liquid suspension is coated continuously onto a current collector followed by a drying process. Usually, current collectors for cathodes are aluminum foils of 12–20 μm in thickness with an adhesion strength of >1 MPa. Ideally, a closed loop handling and recovery of the NMP is realized. A compaction of the cathode is achieved by calendering in a two-roller calender to increase the energy density and conductivity as well as to improve mechanical behavior of the cathode. Typical line speeds for coating and calendering are between 30 to 100 m min−1 and roll diameters of 600 to 1000 mm. Also, a heating of the rollers can minimize an undesired wrinkling of the cathode. Line loads above 1000 N mm−1 for NCM cathodes are described to achieve a 50%-reduction of porosity. Afterwards, the electrodes are usually slitted in width and length. 11 To reduce the water content, a post drying procedure is usually applied before cell assembly. 33 However, if a dry and inert atmosphere is provided a post-drying procedure could be unnecessary. The most important resulting cathode properties are the adhesion strength between the current collector and coating, the stability of the coating, porosity, tortuosity and impedance. 11
Conditioning of the NCM811 particles
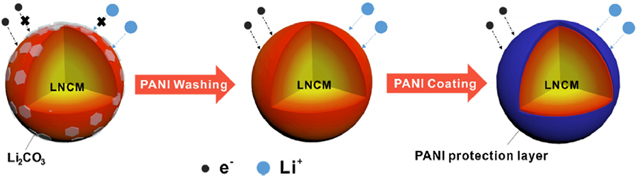
In general, one important property of the active material is the particle size to ensure an advantageous electric and ionic conductivity to realize shorter transportation pathway for electrons and ions. In addition, the particle size affects the quality of the subsequent process steps. For instance, findings suggest that the line load necessary for the electrodes compaction during calendering is determined by the particle size distribution (PSD) and surface. 58 However, in this regard the measurement of PSD is critical and measurement parameters should be chosen carefully for a quantitative statement. In a previous study it is described that a distribution of NCM811 particles with a d50 value of approximately 8 μm is optimal. In comparison to the other tested NCM811 PSDs it also shows a slightly bimodal character. 59 Possibly, narrower NCM811 PSDs are to prefer because of a more homogenous current density. To reduce deleterious surface contaminations caused by humidity and CO2 the NCM811 particles can be washed before the slurry preparation. One possibility is a washing with purified water. However, findings suggest that water washing increases the sensitivity to moisture as well as to CO2 when storing in air which should be avoided especially for NCM811 and is a suspect for increased cation mixing. 53,55 Also, the deteriorating effect of water increases with the washing time. 53 An alternative is washing with protonated polyaniline (PANI) to minimize surface contaminations. However, polyaniline needs a dispersion in NMP before it can be used for the NCM washing. Furthermore, the washed particles also need a dispersion in NMP to remove the polyaniline. If some polyaniline remains in the solution, a coating of the particles could be achieved (Fig. 6). For the PANI washed material a capacity retention of 87% is achieved after 100 cycles at 55 °C and 2 C cycled between 3.0 V and 4.3 V in comparison to 75% for the pristine material. 54
Figure 6. PANI washing and coating of NCM811 active material. Reprinted from Ref. 54. Copyright 2017, with permission from Elsevier.
Download figure:
Standard image High-resolution imageFindings also suggest that NCM811 particles washed with alcohol show a reduced amount of Li2CO3, a higher resistance to CO2 and H2O as well as a reduced cationic disorder. Here a capacity retention of 79% is described for the washed material in comparison to 69% for the unwashed material at 2 C and room temperature after 100 cycles under 4.3 V and after a storage time of 40 d in ambient air. Unfortunately, it is not specified what kind of alcohol is used. 56 Furthermore, the water content should be minimal due to the high sensibility of NCM811 and alcohol of high purity is costly. For NCM622 particles it is described that a washing with phosphoric acid at 80 °C and a subsequent heating to 500 °C could be an effective method to minimize the amount of RLCs on the material's surface. Herein, the acid causes a washing of the RLCs as well as a remaining Li3PO4-coating of the particles which can absorb moisture. 57 Also, a heat treatment can be conducted to minimize surface contaminations. In one study, a heat treatment of 525 °C under an argon flow was implemented for NCM811 particles. In this regard, for NCM811 the temperature must not be too high because of the poorer thermal stability. A sample treated with moisture, a dried sample (120 °C for 12 h) and a calcined sample, which was also exposed to moisture after heat treatment, were compared. In this context, a capacity retention of 55% is described for the wetted material, 85% for the dried material and 92% for the calcined material after 250 cycles at 1 C and 45 °C between 3.0 and 4.2 V. 27 Furthermore, another study provides the hint that cells with LiNi0.80Co0.15Mn0.05O2 being heat treated within an oxygen environment during the material synthesis show a slightly better electrochemical performance than heat treated in ambient atmosphere. The described initial capacity is slightly higher for the material treated with oxygen (194 mAh g−1 and 185 mAh g−1 respectively, cycled at 0.5 C and room temperature). Also, it shows a better capacity retention with 96% in contrast to 93%. 60 Apart from this, during the material precipitation intensive heat treatments are commonly implemented. For example, treatments of >750 °C for 12 h are described. 41,45 Optimally a water and CO2 free atmosphere is provided during the NCM811 production and material storage. In that case an additional washing or heat treatment would be unnecessary before dispersing and parasitic reactions as well as their consequences would be avoided from the process beginning, which is the preferable technology in material synthesis.
Formulation, mixing and dispersion
As for other NCM materials the mixing and dispersion procedure of the active material, binder and conductive additives in a solvent is of high importance for the resulting quality of the NCM811 cathode. It is crucial for the deagglomeration of carbon black as well as for its distribution and structuration within polymer binder phase and on the cathode active material surface. Further overall rheological characteristics of the slurry is of importance. 10,11,14,61–63 The viscosity, for instance, has to be within an appropriate range for the subsequent coating process. 14
For a NCM811 cathode preparation, the composition ratio for the active material, binder and conductive additives given in the literature varies between 80:10:10 and 96:2:2. 64–66 However, the amount of binder should be kept to a minimum because it can contribute as an electric or ionic isolator and decrease energy density 67 and power capability. Furthermore, only formulations with very small conductive additives and binder portions are industrial relevant. Commonly used polymer binders include PVDF. 11 Apart from this, one study reports the achievement of a non-flammable NCM811 cathode due to a fluorinated polyimide binder, which leads to a surface passivation and higher thermal stability. It is described that a NCM811 half-cell with a polyimide binder could be operated up to a cut-off voltage of 4.4 V delivering a capacity retention of 96% in contrast to 4.2 V and a capacity retention of 89% for a cell with a PVDF binder each after 30 cycles and 0.2 C. 68 However, this should be tested for a higher number of cycles.
Ideally, the suspension shows an expressed shear thinning behavior to reach good flow ability of the suspension under shear forces during transport and especially on the slot-die to neglect death zones and demixing effects. Furthermore, a large slope of shear thinning allows to adjust exact edges of the coating. However, the high Ni-content of the NCM811 and surface contaminations affect the rheological behavior of the cathode suspension. As previously mentioned, the higher amount of surface contaminations can lead to a flocculation or gelation of the suspension. In this context, a NMP-based suspension with high stability and without segregation was successfully scaled up to industrial scale by a one-pot approach. For dispersing the NMP was added stepwise to a dry powder mixture consisting of NCM811 as active material, and PVDF binder as well as carbon black (SuperC65) and graphite (SFG6L) as conductive additives and PVDF binder in a planetary mixer. The approach was applied under N2-athmosphere. The declared viscosity is 2.56 Pa s at a shear rate of 100 s−1 and a solids content of 71.3% after 15 h of retention time. Unfortunately, the approach was compared to a multistep procedure with NCM622 as active material. Therefore, the resulting differences cannot be clearly attributed to the different procedures or active materials. 32 In general a systematic approach for investigation of NCM811 cathode material formulation is missing and should be proceeded as Kim et al. showed already in 1999. 69
As NMP is used as solvent it should be water free. Also, the inactive components should be water-free. Furthermore, a water and CO2 free atmosphere within the mixer during dry mixing and dispersing should be prevailed. In this regard, one study reports a better electrochemical performance of a NCM811 cathode whose suspension was stirred in a dry atmosphere (<5% relative humidity). The described capacity retention after 50 cycles is 5% higher compared to a cathode suspension with NCM811 stirred in ambient atmosphere. Both cathodes were post-dryed. 70 This clarifies the importance of the process atmosphere especially during mixing procedure as well as in general during electrode production even if the water content is reduced after post drying. Possibly, the effect onto the electrochemical performance is even stronger during long term cycling, which implies to produce NCM811 under dry room atmosphere.
A more cost-effective, eco-friendly and less harmful alternative to NMP as a solvent for NCM811 containing suspensions is a water based formulation. 13,71,72 A first promising study on aqueous processing shows a capacity retention of 70% after 1000 cycles full pouch cells at 30 °C in comparison to 76% for NMP processed cells. The stronger capacity fading could be attributed to a leaching of Li+ ions. 13 To improve the binding network vapor grown graphite tubes (VGGTs) can be used instead of carbon black. This way, the evolution of secondary cracks within the cathode coating during the drying procedure can be minimized. In addition, isopropanol can be added to reduce capillary stresses. 51 A reduction of the pH-value by acid addition to diminish the corrosion of the aluminum foil is another consideration. 73 The evolution of cracks within thicker water-based NCM811 coatings and its prevention is further specified a following section. Besides, it is reported that aqueous processed NCM523 cathodes show a higher water uptake in comparison to NMP processed cathodes even if post-drying could reduce the residual water content on a similar level. 74 However, because there is a highly intensive contact to water during the electrode processing which leads to parasitic side reactions on the NCM811 particle surfaces, adaptions have to be applied like a NCM811 coating. Apart from this, the rheology as well as the agglomeration and sedimentation behavior of aqueous NCM811 cathode slurries should be investigated.
Besides, a small amount of LiPF6 (0.5–1.0 wt%) could be added after feeding the dry components into the solvent like it is described for a NCM811 suspension. The acidic LiPF6 reacts with RCLs resulting in Li3PO4 and LiF which are components of a stable electrolyte interphase. 75 The resulting CO2 and hydrofluoric acid (HF) are removed during the drying process. 6 However, it should be noted that HF can dissolve the transition metals within the active material and is highly corrosive and acute toxic. 76 Moreover, a patented method for an NCM811 electrode manufacturing in which a polymer electrolyte is also contained in the cathode slurry exists. 77
Summarized, the process atmosphere as well as the raw materials should be water-free in case of a NMP-based suspension. For an aqueous processing of NCM811 cathodes the environmental, health-related and economical aspects as well as the effort to overcome the detrimental effects of water have to be taken into account.
Coating and drying procedure
In general, the liquid suspension is coated onto the current collector and the solvent is removed by a drying process under defined conditions. Herein, the result of this process is largely dependent on the properties of the suspension and the drying conditions along the dryer length. 11 Besides, it affects the adhesion strength between the coating and current collector as well as the cathode's conductivity 14 due to segregation effects to binder and carbon blacks. 78 Usually the cathode slurry is coated on an aluminum foil. 11 Conventional electrodes are based on liquid suspensions. During aqueous processing, a copper foil could prevent the evolution of hydrogen and hence the origin of cracks within thicker NCM811 cathodes during the drying process. 51 However, different studies report a solvent-free dry powder coating process that is a time-efficient, environmental friendly and less harmful alternative 50,79–81 to ensure establishment of binder network. The powder mixtures are sprayed electrostatically or bulk material is dosed and is fixed onto the current collector by hot pressing, hot rolling or heating in a convection oven at room temperature. 50,79–81 This way, the process duration could be shortened and the exposure to liquids like NMP and its recovery is avoided. 79 Although a solvent-free production of NCM811 cathodes is to our knowledge not established yet, it is a highly interesting approach because there is no contact to liquids. However, the dry mixing procedure has to be adapted similar to the way it is described for dry anode materials. For example, a large particle size of the binder compared to the active material particles is unfavorable for the dry mixing resulting in an unstable coating. 50 Another study describes a reduced decomposition of the electrolyte due to a thiophene-initiated artificial cathode electrolyte interfacial (CEI) layer coated onto the NCM811 cathode surface after the calendering. Herein, the molecular weight of the polymer should be low because of a better thermal stability originated from its higher crystalline order. In this context, the effect of the polymer coating is more evident at higher temperatures. The described capacity retention for a cathode with a 36 kDa polymer coating after 50 cycles at 1 C and 55 °C is 89% in contrast to 55% for a cathode with bare NCM811 material. 82
Like for other cathode active materials, the subsequent drying has a strong impact on the cathode properties. Drying is necessary to evaporate the solvent and to establish a polymer-particle network to form a solid coating on the substrate. In case of a dry coating process usually a heat treatment is required to establish a binder network as well. 80 During the drying process, solvent evaporation and particle sedimentation were found to potentially cause demixing and result in a concentration gradient of the binder and carbon black, leaving an increased binder and/or carbon black concentration at the electrode surface as illustrated in Fig. 7. 12,83–87
Figure 7. Schematic illustration of solvent evaporation and particle sedimentation as main driving forces of demixing during electrode drying. Republished with permission of IOP Publishing, Ltd. from Ref. 83. Copyright 2015, permission conveyed through Copyright Clearance Center, Inc.
Download figure:
Standard image High-resolution imageDemixing is an unwanted effect as it hinders the formation of the binder-particle network and therefor reduces adhesion strength and increases electrical resistance of the electrode. 12,85,86 These findings from LiCO2, 86 NCM111 85 and NCM622 cathodes 12 are likely transferable to cathodes with NCM811, hence physical process conditions and material properties stay the same. However, no investigation of optimal drying parameters specifically for NCM811 coatings have been to our knowledge published yet. Furthermore, the drying is strongly affected by the chosen solvent. Therefore, it is necessary to find optimal drying parameters for both separately. While the drying process of water-based NCM811-coatings has been investigated more closely, 71,88,89 there are no studies specifically on drying procedures and parameters for NMP-based NCM811 cathode coatings to our knowledge. To evaluate specific challenges of NCM811 active material in drying of NMP-based coatings further investigations, preferably in comparison to water-based formulation, are needed.
It was suggested by Wood et al. that if water is used as solvent instead of NMP costs of the electrode processing (drying and solvent recovery steps) could be cut in half and that the process equipment invest could be further reduced. 71 NMP has a higher boiling point than water (NMP: 204.3 °C and water: 100.0 °C at ambient conditions) and a very low vapor pressure (NMP: 1.0 mmHg, water: 55.3 mmHg at 40 °C) which causes high energy consumption during the drying of NMP-based slurries. 71 Additionally, higher solid loadings can be achieved with water-based slurries which can reduce the retention time especially for the drying of thick electrodes. 71 Wood et al. calculated that for an anode baseline case in total about 25.7% of the energy needed for the solvent evaporation could be saved if water was used instead of NMP. 71 Emission control and solvent recovery equipment to keep the NMP concentration in the air below 0.325 vol% is mandatory for explosion prevention which causes higher investment costs. 71 Furthermore, material cost savings of about 80% have been estimated for water-soluble binders like carboxymethylcellulose (CMC), styrene butadiene rubber (SBR) for CMC/SBR compared to PVDF binders. 90 These findings suggest that using water-based slurries in electrode production can reduce the price per usable kWh significantly which is one of many reasons making water-based slurries attractive for the industry.
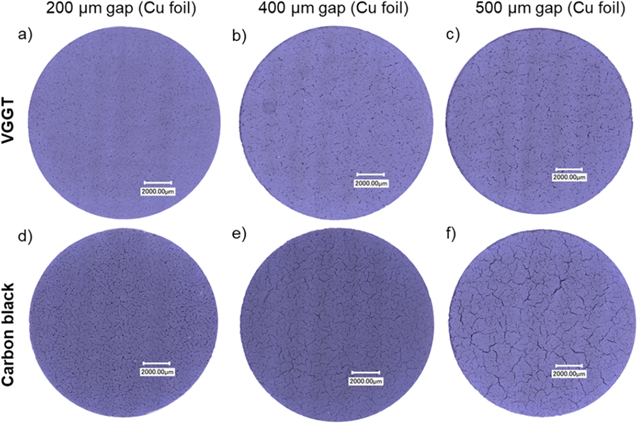
On the other hand, water based NCM slurries tend to develop more defects during coating and while drying. 91 One reason is the high pH-value of water based NCM slurries, which is caused by the cation exchange of Li+/H+ and the following formation of LiOH in the slurry. The native pH-value in aqueous dispersions of NCM111 is 11.3 while for NCM622 and NCM811 it is 12.3 and 12.4, respectively. 55,73,92 Higher pH- values in NCM811 are therefore likely to worsen these detrimental effects, especially aluminum corrosion. The oxide layer on the aluminum foil is only stable for pH-values of 4.5 to 8.5. With increasing pH-values the oxide layer is dissolved and the aluminum is exposed to the slurry resulting in further degrading processes. 88 The corrosion of aluminum leads to the formation of water soluble aluminum species, pitting of the aluminum foil and the release of hydrogen which produces cavities, leads to cracks and defects, and causes demixing of active material, binder and conductive additive in the cathode. 51 The pH-value of aqueous slurries generally correlates with the capacity of the cathode active material which increases the severity of corrosion effects. To prevent these defects multiple measures have been identified. One of these methods is the addition of acids to the slurry in order to lower its pH-value. While the corrosion can be reduced when acids are used to reduce the pH-value and even fully prevented when the pH-value is lowered beneath 8.5, the adding of acids comes with other negative effects regarding the rheology of the slurry as well as the conductivity and adhesion strength of the coating. 73,89 For thick water-based NCM811 cathodes an addition of 1 wt% of phosphoric acid was found to eliminate aluminum corrosion and improve the formation discharge capacity and high rate discharge capacity of the cell. 89 While the formation capacity could be raised by 20 mAh g−1 and 10 mAh g−1 for thick NCM811 cathodes with an areal capacity of 6 mAh cm−2 and 8 mAh cm−2, respectively, the differences in high rate discharge capacities remained minor. 89 This goes in opposition to findings for aqueous processed NCM111 cathodes for which the reduction of the pH-value to 9 using acetic acid led to an increase in discharge capacity during 100 cycles at 1 C of about 20%, while the adding of poly acrylic acid reduced the discharge capacity. 73 If the differences in the effect of acid addition on the discharge capacity for aqueous processed NCM811 and NCM111 cathodes is related to the Ni-content of the active material or emerges due to the use of different acids needs further investigation. For both active materials and acids used, downsides have been observed. With the addition of phosphoric acid to the aqueous-processed NCM811 cathodes, the adhesion dropped significantly. 89 The better adhesion for slurries with high pH-values are most likely due to invasion of the slurry into the pits caused by the corrosion of the aluminum substrate. The phosphoric acid alters the rheology of the acid with an increasing effect on low-shear viscosity(LSV) as well as high-shear viscosity(HSV) up to 1 wt% and 0.75 wt% and a reduction in both LSV and HSV for higher phosphoric acid contents. Although at 1 wt% addition of phosphoric acid the agglomeration tendency is highest, this concentration yields the best electrochemical and mechanical properties. 89 A reduction to pH 9 in NCM111 cathodes using acetic acid was found to result in only a moderate increase of slurry rheology and minor impairment of electrode conductivity and adhesion strength. 73 These downsides of acid addition need to be kept in mind for further investigations on pH-reduction for aqueous processed NCM811 cathodes. Another measure to counteract corrosion can be the coating of the current collector or the active material particles, which however, comes with drawbacks like an increase of resistance. 88,93 Coating the active material particles can also be a suitable means to mitigate the dissolution of transition metals. 93 It has been suggested that a two-step approach of a thin coating of active material particles for reduction of transition metal leaching and a moderate pH-reduction to 9–10 using acids for mitigating corrosion might be a suitable approach. 73 Though, to our knowledge, there is no study elaborating this approach yet. Another reason for defects of cathodes made from aqueous NCM slurries is the high surface tension of water. A reduction of cracks and defects in NCM811 cathodes made from aqueous NCM811 slurries was observed when using vapor grown graphite tubes (VGGTs) instead of carbon black as conductive additive. In Fig. 8 the influence of VGGT as conductive additive on the amount and magnitude of cracks in the cathode surface is shown in an optical micrograph. Especially, with respect to thick coatings beneficial effects of VGGTs on the coating quality can be noticed. 51 The authors argue that VGGT improved the distribution of binder and increased the cohesion of the coating.
Figure 8. Optical micrographs of aqueous processed NCM811 cathode coatings on a copper-foil with different doctor-blade gaps and different conductive additives, (a)–(c) VGGT or (d)–(f) carbon black. Reprinted with permission from Ref. 51. Copyright 2020, American Chemical Society.
Download figure:
Standard image High-resolution imageFurther, adding 12 wt% of isopropanol (IPA) as co-solvent was found to be a suitable method to decrease coating defects. In Fig. 9 the influence of the coating thickness and the use of IPA as co-solvent on the coating quality and defects is shown under an optical microscope. The authors argue that IPA reduced the surface tension and capillary stress and therefore reduced cavities in the coating. Both, the use of IPA as co-solvent and the use of VGGT as conductive additive led to a significant decrease in cracks of the coating. 51
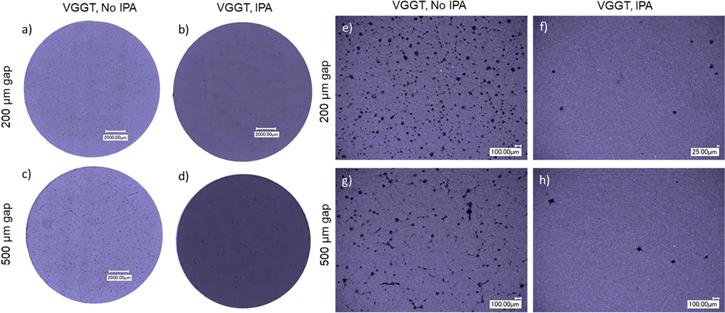
Figure 9. Optical micrographs of aqueous processed NCM811 cathode coatings on a copper-foil with VGGT as conductive additive with different doctor-blade gaps and different solvents (NO IPA equals 100% water, IPA equals water/IPA-ratio of 88/12). Reprinted with permission from Ref. 51. Copyright 2020, American Chemical Society.
Download figure:
Standard image High-resolution imageCalendering
Calendering was found to have a significant effect on electrode properties due to its influence in porosity and density. 94–96 While a porous electrode is needed to ensure the transport of lithium-ions to and from the active material particles, a denser electrode has an improved electron conducting network. 58,94,97–99 Especially for electrodes with low concentration of conductive agent a dense electron conducting network is beneficial for the electrochemical properties of the LIB. Compression of NCM cathodes can increase the adhesion strength of the coating on the current collector. 49,94
However, when calendered to medium porosities of 30%–35% the adhesion strength drops significantly. Due to different elastic moduli of the PVDF-binder (approximately ≤ 1 GPa) and the aluminum-substrate (70 GPa) while calendering the lateral stretching is higher for the coating. That causes detachments in the interconnections of the binder to the substrate which decreases the adhesion strength. Strong calendering to porosities lower than 30% (minimum porosity was achieved at 18%) increases the adhesion strength due to mechanical interlocking of the substrate with the coating particles. 49
A method to improve the adhesion strength is to increase the temperature of the calender rolls. As one reason one could mention that an increased temperature softens the aluminum substrate and improves the mechanical interlocking of the substrate with the coating particles. Additionally, the low adhesion strength at porosities of around 30%–35% can be mitigated with higher binder contents. 49
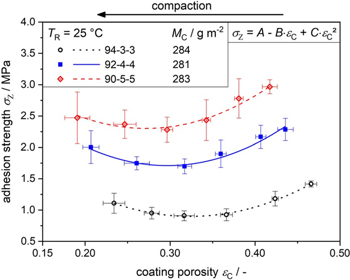
As seen in Fig. 10, for NCM111 cathodes with mass loadings of 280 g m−2, a mixture with a composition of active material to binder to conductive additive of 94:3:3 had a minimal adhesion strength of ca. 0.9 MPa at around 32% porosity. In contrast, a mixture of 90:5:5 had a minimal adhesion strength of ca. 2.3 MPa at a porosity of 28%. 49 Also, the initial electrode porosity as well as the minimum porosity after calendaring decreases with higher contents of binder and conductive additive. Similar results have been observed for less thick cathodes. NCM111 cathodes with mass loadings of 160 g m−2 and a mixture of 94:3:3 had an initial porosity of 47% and a minimum porosity of 25%, whereas a mixture of 90:5:5 had an initial porosity of 42% and a minimum porosity of 18%. 49 Therefore, calendering parameters have to be adjusted to specific contents of binder and conductive additive, as well as the mass loading of the electrode.
Figure 10. Adhesion strength σZ of NCM111 cathodes of different compositions at constant mass loading MC and constant roll temperature TR under the influence of the calendering. Copyright 2019, Wiley-VCH. Used with permission from Ref. 49.
Download figure:
Standard image High-resolution imageAnother factor that needs consideration is the effect of the high mechanical stress during calendaring, especially for cathodes. Strong calendering has been shown to cause cracks in the cathode active material particles. 100–102 The packing density of the NCM active material particles has been suggested to be the limiting factor for this effect. 103 The active material particles absorb the compression energy and crack as soon as no further repositioning under compression is possible. Also, strong calendering can cause fractures in the electrode coating which inhibits electronic conductivity through disruption of conductive pathways. 100 The particle size of the active material and its distribution mainly influences the compaction resistance, which is caused by differences in friction during rearrangement of particles during compression. 58 Results from analyzing the calendering of NCM active material particles with different Ni-content by Meyer et al. indicate that higher Ni-content might be related to reduced breaking strength, although further research is needed to rule out the influence of the production methods on observed differences in breaking strength. 58 Lower breakings strengths could lead to increased particle cracking during calendering. Depending on the applied pressure, undesired folds can develop at the intersections of uncoated and coated foil due to differences in stretching. 104 Additionally, the optimal amount of compression is depending on previous process steps. The mixing of the cathode slurries and the agglomeration state of the conductive additive influence the required compressing. 58 These complex and partly interacting mechanisms increase the importance of finding the optimal degree of compression in order to achieve desired electrode properties.
An investigation of different cathode active materials including NCM111, NCM622 and NCM811 of very similar particle size found similar optimal porosities for the different active materials which were mainly influenced by desired discharge rate and adhesion strength of the coating on the current collector. A porosity of not more than 36% has been found to be needed for establishing conductive pathways in NCM811 cathodes. 105
Post-drying
To avoid material degradation and detrimental effects on battery cell properties it is important to reduce the amount of residual moisture in the battery cells before assembly. 11 The post-drying process is normally either carried out before or after calendaring to ensure that the residual moisture in the electrodes is sufficiently low during cell assembly which takes place in dry room atmosphere. 11 Another approach is to realize the whole NCM811 electrode production chain, beginning from the mixing process, within a dry room. Since the latter is the safer but more expensive alternative, knowledge according this topic has to be generated.
However, residual moisture hydrolyses the conductive salt LiPF6 under formation of gaseous HF. The HF causes structural damages due to the volume expansion and causes further degradation reactions in the cell. 6 In general, the sensitivity to moisture rises with increasing Ni-content of NCM materials. 27 Therefore, adequate post-drying or complete dry room processing is more important in Ni-rich cathode active materials. However, it has been shown with NCM622 cathodes that an intense post-drying routine can get in conflict with a good cell performance due to heat induced diffusion creep in the binder matrices and further degradation processes. 33 Furthermore, small amounts of residual water support the formation of the SEI during cycling which increases the cycle lifetime and are therefore necessary to increase the cycling stability. 34 The fine adjustment of post-drying parameters with respect to the specific material properties is of great importance to ensure the best outcome for the battery cell properties. To our knowledge no research has been conducted to determine optimal post-drying conditions for NCM811 cathode active material. A study on optimal post-drying parameters for NCM622 demonstrates the importance of the post-drying procedure for the improvement of battery properties. The study showed beneficial outcomes for the properties of NCM622 cathodes using a less intensive post-drying method by purging with argon atmosphere at only 20 °C. 33 Even though the residual water content in the full electrode-separator-assembly (ESA) was higher using these post-drying parameters (Argon post-drying (APD): 15 min for 20 °C led to 326 ppm, compared to vacuum post drying (medium-Vac.-PD): 18 h 120 °C led to 225 ppm, and vacuum post-drying (long-Vac.-PD): 96 h 120 °C led to 136 ppm), the mechanical and electrochemical properties improved, supporting the notion of more complex mechanisms underlying the post-drying process. 33 In principle this shows that microstructural changes induced by intensive post-drying affects the performance in a greater manner than a residual moisture content of 336 ppm. In detail, the specific discharge capacity during the different cycling steps of this study are presented in Fig. 11. The electrochemical investigation consisted of phases of three cycles each with different discharge rates of 0.1 C/0.2 C/1 C/2 C/3 C/5 C, followed by two recovery cycles at 0.1 C before long term cycling stability was tested with a discharge rate of 1 C over 50 cycles. This procedure was repeated three times. In this investigation, the specific discharge capacity of the APD treated NCM622 cathode was 40% higher during the 50th cycle and even 145% higher during the 200th cycle than that of the long-Vac.-PD treated cathode at a discharge rate of 1 C, which underlines the importance of finding suitable parameters as a trade of between microstructural change and residual moisture. 33
Figure 11. Specific discharge capacities of NCM622-graphite one-layered pouch cells resulting from different post-drying procedures with indication of the remaining water content in the entire ESAs. Average of three samples each. Copyright 2019, Wiley-VCH. Used with permission from Ref. 33.
Download figure:
Standard image High-resolution imageIn industry post-drying is commonly performed in a vacuum oven as batch drying process or as coil-to-coil drying process often with infrared, which makes these results only partly applicable, but microstructural changes have to be taken into account anyways. 11 Another advantage of the APD method is the short duration of only 15 min compared to Vac.-PD methods that can last 10 h and longer. 33 As previously mentioned, NCM811 reacts more sensitive to residual moisture, which possibly makes intensive post-drying processes advantageous for NCM811 cathodes. Further research is needed to extend the findings for NCM622 to NCM811 and to verify the beneficial effect of more intensive post-drying procedures for the battery properties. Additionally, the necessity of a totally controlled production environment when proceeding all process steps in a dry room needs further investigation.
Conclusions
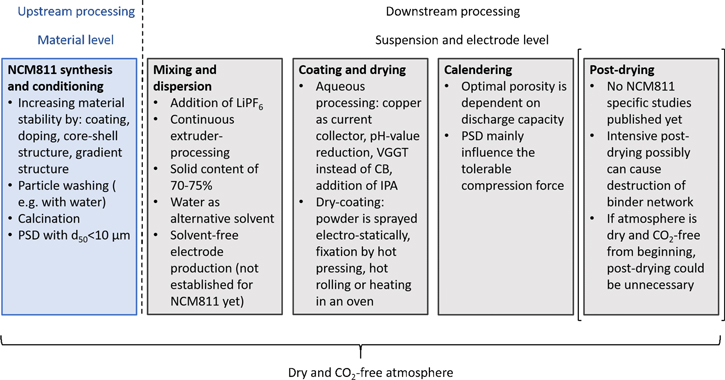
The main challenges to establish an effective production chain for cathodes with NCM811 as promising active material are to overcome performance degradation and safety issues due to surface contaminations, the loss of lattice oxygen, and cation mixing. According to the current state of knowledge the process atmosphere, solvent and material surfaces should be kept water and CO2 free to avoid parasitic side reactions and to ensure long term stability. Apart from this, possibilities to stabilize the material are a surface coating, doping with foreign ions, gradient or core–shell structures. A washing or heat treatment can be implemented to reduce surface contaminations caused by humidity and CO2. Here, the water content of the washing agent should be kept to a minimum. Furthermore, a solvent-free coating process is a highly interesting approach to produce NCM811 containing cathodes because a contact with liquids is avoided. Therefore, a dry powder coating process should be considered in future studies for NCM811. Compared to this, an aqueous processing needs various process adaptions to avoid a corrosion of the current collector, the evolution of cracks during the drying procedure within thicker NCM811 cathodes as well as the detrimental effects of water-contact. Besides, like for other NCM materials drying can lead to a demixing of particles in the coating when drying temperature is too high, it is necessary to use a temperature adapted not only for the used materials, but also for the specific cathode thickness. Research on the calendering of NCM811 suggests that a porosity of not more than 36% is needed to ensure needed feasible electrical conductivity. Studies on optimal post-drying parameters of NCM811 cathodes are needed. However, investigations on related materials suggest mild post-drying processes can prevent damage to the binder and therefore increase mechanical stability and electrochemical performance indicating that reducing residue moisture cannot be the only focus of the post-drying process. As the research on process parameters for NCM811 cathodes is very limited until today, further research is needed to validate the estimated optimal process parameters. Figure 12 provides an overview of the possibilities and process approaches discussed in this review.
Figure 12. Possibilities discussed in the literature to improve cathode production chain with Ni-rich NCM as active material.
Download figure:
Standard image High-resolution imageAcknowledgments
The authors gratefully thank the German Federal Ministry of Education and Research (BMBF) for the financial support (03XP0247A) and Laura Jess for her comments on the review article.