Abstract
Green energy harvesting (solar and wind) and storage along with electrification of transport sector could bring about a major transformation in the CO2 emission levels that we are currently experiencing. Lithium ion batteries provide an efficient energy storage system to realize this goal. The key developments in Li-ion battery technology starting from solid solution electrodes, intercalation electrodes, conversion electrodes, organic electrodes, and polymer electrolytes with a major focus on the contribution of Michel Armand, an eminent scientist who at a young age saw the future of energy storage, have been elaborated. Moreover, the direction of research that seems interesting to pursue for realizing our goals has also been outlined.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Our current scenario on global warming impels the reduction of utilizing fossil fuels such as oil and gas. Renewable energies (wind and solar energy) seem to be a better alternative in order to diminish CO2 emissions that result in climatic changes and global warming. On the other hand, electrification of transport sector that accounts for nearly 14% of the CO2 emission could be an effective strategy.1 Renewable energy harvesting is not an easy task due to the lack of continuous supply and is highly dependent on climatic conditions. An efficient technology for renewable energy storage that facilitates use-on-demand is currently the need of the hour. High-performance rechargeable batteries have become so popular in the past decades that it has nearly become a household word. Realizing the importance of the aftermath of global warming, development of efficient green energy harvesting and energy storage technologies has been rapid, which in turn has increased the popularity of this field.
Energy transition scenario
Many countries are in the midst of transition from biofuels to electricity. Electricity when harvested from renewable source is considered as clean energy. Electricity accounts for 1/5th of the total global final energy consumption that experiences an increase in consumption in high-income countries.2
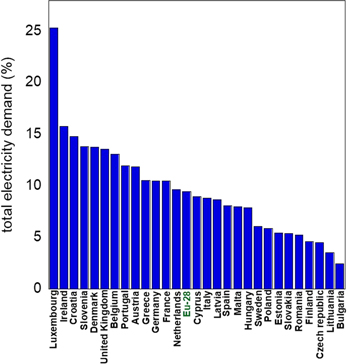
Estimation by the EEA (European Environmental Agency) suggests that the measures taken to reduce greenhouse gas emission will have an impact on the European Union's energy consumption. Assuming that 80% of cars would be electrified in 2050, the EU-28 member states (Fig. 1) would experience an increase in total electricity demand that would vary between 3 and 25% in 2030 and an average of 9.5% in 2050.3 This leads to an additional required electrical capacity of 150 GW for charging electric cars.
Figure 1. Electric vehicle (EV) energy demand as a percentage of total electricity demand in 2050.3
Download figure:
Standard image High-resolution imageSome of the renewable energy targets in other countries have been increased foreseeing the growing demand aimed at electrification of transport sector. The renewable energy target for India due to its good progress has been raised to 227 GW by 2027 wherein a major part is contributed from wind and solar energy.4 According to the U.S. Energy Information Administration, renewable energy accounts for 11% of the total energy demand and 17% of all electricity generation.5 China being the largest energy producer and consumer has set a target in total energy demand supply from renewables (non-fossil fuel share) to around 20% by 2030,6 with the Russian federation already crossing its 2024 target of 5.9 GW from renewable energy (including geothermal).7 The energy transition from biofuels to renewable could be effective with efficient energy storage systems (short- and long-term storage) that would bring us closer to our goals of reducing CO2 emissions with complete transformation in the transportation sector to EVs.
Status of lithium batteries
During the past decades, the advent of portable electronics led to the increase in demand for battery systems. Different energy storage systems were under consideration before the development of lithium secondary batteries, for instance, nickel metal hydride, lead acid batteries, redox flow batteries and fuel cells. However, the low energy density, power density and storage efficiency seemed to limit their potential for further developments. Lithium was seen as a candidate of choice due to its highly reducing nature (−3.04 V vs standard hydrogen electrode), small atomic radius (high diffusion coefficient), and low atomic mass. Lithium ion batteries became a market boomer for portable electronics since their commercialization by Sony Corporation in 1991.
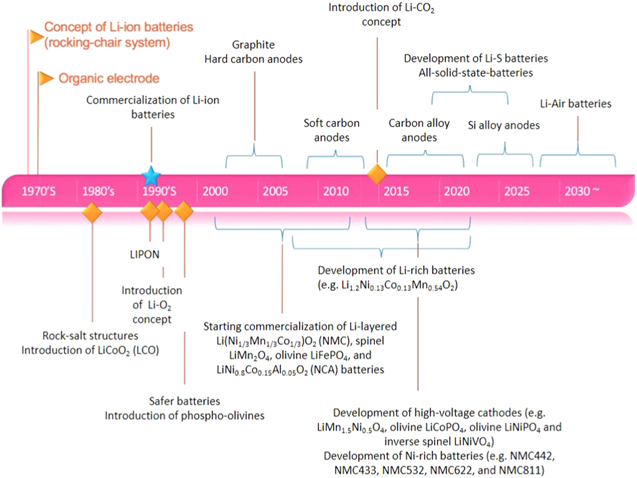
The intermittence or discontinuous nature of renewable energy sources requires high efficiency energy storage systems. Electrochemical systems, like batteries and super-capacitors, play a crucial role in this field. They provide efficient storage and delivery of energy, either on demand in stand-alone systems or load leveling in electrical grid integrated systems. When compared to lead acid batteries, lithium ion batteries are also seen as the power sources of choice for sustainable transport as they are considered the best option that can effectively guarantee the progressive diffusion of HEVs, PHEVs, and BEVs at high levels. The developments in lithium battery technology are shown in Fig. 2, starting from the introduction of rocking chair Li-ion batteries, organic electrodes, to state-of-the-art solid-state batteries and post Li systems such as Li air batteries. Many scientists have contributed their part in the development of materials and components for lithium batteries of today.
Figure 2. Historical evolution and advances of lithium battery technologies.8
Download figure:
Standard image High-resolution imageHerein we focus mainly on the marked breakthrough findings that ushered the rapid development of lithium-ion/lithium metal battery technology till date, through intercalation, conversion, organic electrodes, and polymer electrolytes where innovative concepts have been introduced by an outstanding scientist Michel Armand, with a brief outline of other similar improvements.
Intercalation Electrodes (Evolution and Progress)
Early studies by B.C.H. Steele showed that host materials that could accommodate electroactive species like hydrogen, oxygen, copper, lithium, and sodium, which possess high solubility and rate of dissolution, could be used as solid solution electrodes (SSE).9 An emphasis was made on TiS2 by Steele and co-workers (transition metal dichalcogenide) for its use as positive electrode materials in lithium batteries in 1972.10 Later, in 1976, works carried out by Steele et al. and Whittingham et al. proved the rapid intercalation and reversibility of Li ions into TiS2 with a Li metal/TiS2 cell.11–14 Parallely, Michel Armand, in 1972, proposed the property requirements of materials for an intercalation electrode in a conference at Belgirate, Italy.15 He showed, for the first time in a solid-state setup, Na+ diffusion in ternary graphite intercalation electrode (C8@CrO3) using β-alumina as electrolyte corresponding to the overall reaction as given below.15

The first battery tests (Fig. 3a) using a striated β-Al2O3 electrolyte (to increase the contact as well as to prevent removal of Na metal) were carried out at 25 °C at 0.1 mA cm−2. When β-Al2O3 was replaced by a solution of PC and NaClO4, some issues-related Na metal corrosion and insertion of solvated species were observed.
Figure 3. (a) Schematic of first solid-state experimental battery setup15; (b) Li metal anode (top) and Li intercalation host as anode16 (bottom); (c) first experimental results of a full Li intercalation host-based LixWO2/LiyTiS2 rechargeable cell17; (d) schematic of carbon coating on LiFePO4 particles18; (e) CV scans of uncoated and carbon-coated LiFePO4 electrodes18; (f) concept of hindered glymes for graphite electrodes/structure of hindered glyme molecule19; (g) cycling tests with Li metal/graphite electrodes.20
Download figure:
Standard image High-resolution imageIn the same presentation, he also discussed that although only Na+ is able to intercalate, with different electrolyte systems it is possible to intercalate Li+ and K+. This could be realized by thin-film polymer membranes or even with cathode soaked in a polar liquid for better contact. The polar liquid is required to possess enough steric hindrance that could prevent the solvated species from entering into the planes of the graphite C8CrO3 positive electrode. Although electrochemical anion intercalation was first probed by Schafhaeutl21 in 1840, electrochemical cationic intercalation was proposed by Michel Armand.
At this juncture, it is worth mentioning that based on the concept foreseen by Michel Armand on steric hindrance, to prevent solvent co-intercalation, years later in 2015, he introduced the concept of using "hindered glymes" as graphite-compatible electrolytes.19 His ideas were focused on employing glymes as safe solvents for electrolyte composition but at the same time prevent solvent co-intercalation. A new electrolyte composition was proposed comprising a polyether to which a bulky tertiary group was attached that prevented co-intercalation into the graphite while still maintaining high conductivity. A schematic of the concept and the structure of the custom-synthesized glyme molecule are shown in Fig. 3f. The cells when cycled with graphite electrodes showed stable cycling capacity (Fig. 3g) when compared with EC/DMC/LiFSI and marked performance enhancement when compared with a commercially available di-propyl glyme-based solvent.20
Rocking chair systems
TiS2 intercalation electrode was the most successful intercalation cathode material with a layered structure that received wide attention, although safety issues due to dendrite formation while employing Li metal was a major drawback. In the late 1970s, Michel Armand proposed the use of intercalation host materials with different potential as both positive and negative electrodes that formed a major breakthrough for the rise of the "rocking chair concept" rechargeable batteries.22 In these types of rechargeable batteries, the Li+ ions reversibly intercalate between the positive and negative electrodes (Fig. 3a bottom), thus circumventing the issue of Li dendrite formation as shown in Fig. 3b (top).16
First experimental results for rechargeable cells with two lithium intercalation host materials were carried out by M. Lazzari and Scrosati using LixWO2/LiyTiS217 system. Promising results were shown (Fig. 3c) that attracted attention from many corners towards the development of "rocking chair" rechargeable batteries.
In 1980, Goodenough et al. proposed a new type of cathode based on layered oxide (LiCoO2) that unveiled a doubled up potential when compared to TiS2 and good electrochemical reversibility.23,24 Until today these materials are employed in lithium batteries. In addition, Goodenough also proposed a spinel material lithium manganese oxide (LiMn2O4) in 198025 and then an olivine material LiFePO4 in 1996.26 The olivine LiFePO4 was of high interest due to the natural abundance of iron as well as due to being safe and cost effective. The only drawback that prevented it from commercialization during that time was its low electronic conductivity.
The pioneering work by Michel Armand and co-workers on carbon-coated LiFePO4 cathode materials18 improved their performance considerably. A polymer precursor (organic polymer) was thermally decomposed and a uniform layer of carbonaceous layer was formed on the surface of the particles (Fig. 3d).18 This layer accounts for a regular distribution of electric field on the surface and diminished ion concentration gradients thereby improving the kinetics in terms of power and current density. Cyclic voltammetry tests (Fig. 3e)18 showed improved kinetics when compared to uncoated LiFePO4 samples. The once considered dead-end material regained its popularity worldwide making it a candidate of choice for use in lithium batteries currently.
Conversion reaction electrodes
Li-ion batteries based on intercalation type of electrodes have stable cycling performance and low self-discharge. One of the main drawbacks of the intercalation electrodes is the low specific capacity. This diverted the attention towards transition metal oxide electrode materials that seemed promising as a replacement to the intercalation electrodes. Metal oxide based lithium-ion cells were proposed to have twice the capacity when compared to LiCoO2/C cells due to the metal oxide 2-fold capacity per unit mass and 3-fold density, leading to 6 times increase in capacity per unit volume when compared to carbon. Therefore, a multi-electron reaction with phase nucleation ("conversion reaction"), e.g., TMO + 2Li+ + 2e−⇆ TM(0) + Li2O (TM = Co, Ni, Mn...), was proposed.27–30 Several materials like transition metal oxides and sulfides were utilized as high capacity anode materials, and some transition metal fluorides have shown great potential as cathode materials for Li rechargeable batteries with a high theoretical capacity ranging from 500 to 1500 mAh g−1.31 Although conversion reaction electrodes seem to be an impressive alternative, the reconstruction of TMO from TM(0) + Li2O in such electrodes is only partially reversible and, additionally, a large amount of Li+ are consumed in the build-up of SEI layer. Both processes are responsible for the first cycle irreversibility of around 30% which leads to a major concern for the development of these materials.
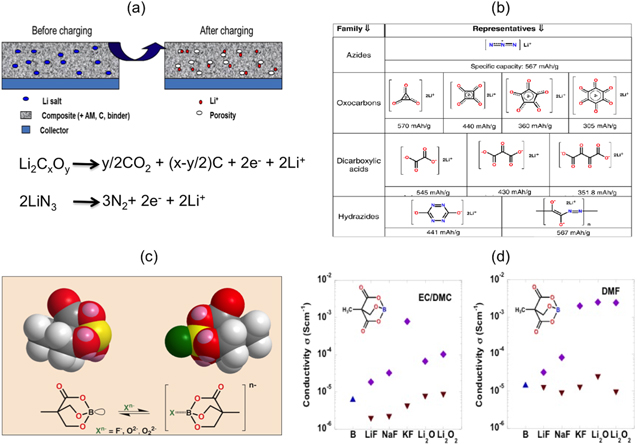
Michel Armand et al. proposed a facile, innovative, and efficient solution to this issue. Firstly, the route to solve the first cycle irreversibility was suggested by incorporating sacrificial salts, i.e., salts with an oxidizable anion that delivers the extra lithium required for the battery system. The schematic of the concept of sacrificial salt is shown in Fig. 4a.32 The salt is incorporated into the cathode as an additive. During charge, the salts get oxidized to supply extra lithium, and the proposed reaction mechanism is shown in Fig. 4a. A by-product of the reaction is the formation of porosity that could facilitate better electrolyte wettability.
Figure 4. (a) Schematic of the concept sacrificial salt and the overall reaction of Li2C4O4 and LiN3 sacrificial salt32; (b) structural formula and specific capacities of the compounds corresponding to the four salt families33; (c) schematic of tunable anion carriers and the trapping mechanism; (d) ionic conductivities with and without of Boron di-acid additives.34
Download figure:
Standard image High-resolution imageThe salt families that could be utilized were identified as listed in Fig. 4b.33 According to the requirements of the anode employed, the sacrificial salts can be chosen based on their oxidation potential for use with different anodes. The versatility of this concept is that in addition to conversion-based electrode, these salts can also be used for Li-ion batteries employing graphite as anodes as well as extended to any metal ion battery system (e.g., Na, K, etc.).
Controlling the SEI is of utmost importance for safety and cycle life of the cells especially in conversion reaction electrodes. Excessive thickness results in irreversible capacity loss and increased impedances. The large specific capacities obtained are appealing, but the charge and recharge processes are separated by almost a volt, reducing the energy efficiency. Therefore, in order to bring about partial solubilization of Li2O, tunable anion carriers35 were synthesized that could bypass the rate-limiting diffusion path in the solid state, and thus reduce polarization. The mechanism of boron complex additives that can trap anion, thus favoring partial solubilization, is shown in Fig. 4c. Ionic conductivity tests with the boron complex additives and other insulating salts like LiF, Li2O, etc., in EC/DMC or DMF solvent, show an improvement in ionic conductivity (Fig. 4d),34 thus showing the practicality of such additives. These additives could also be employed for Li-ion batteries in order to have a partial solubilization of LiF formed in the SEI, thus reducing the overall impedance of the cell.
Organic Electrodes
Li-ion batteries using intercalation electrodes are the predominant battery technology to date. Due to their high specific energy and energy efficiency, these batteries got an immense success in portable electronics and are considered for EVs, as well as grid energy storage. However, further employment of these types of cathodes, notably for EVs, seems unpractical for two main reasons. Firstly, the use of conventional cathodes based on cobalt and nickel is unsustainable due to the scarcity and toxicity of these elements. An overexploitation of these transition metals may also lead to severe environmental and geopolitical issues.36 Secondly, though other intercalation electrodes are optimized (e.g., polyanionic materials), safety concerns remain, as the limited flexibility of ceramic-based electrodes can lead to dramatic events under high mechanical stress in combination with a flammable liquid electrolyte.37
To overcome these issues, electroactive organic materials are considered to be one of the best alternatives. Organic materials are intrinsically softer and less prone to swelling upon cycling when compared to crystalline structures of intercalation electrodes, allowing the fabrication of design flexible and safe batteries.38 Furthermore, its rich chemistry and abundance (bio-sources) make these materials appropriate for obtaining cheaper batteries.39 Organic materials have high theoretical specific capacity, longer cyclability, and faster kinetics than inorganic ones; however, low working voltage, possible dissolution in the electrolytes, low volumetric capacity, and poor electronic conductivity are the main drawbacks of some of these materials. Nevertheless, since organic electrode materials are highly and easily tunable, we can overcome most of these limitations by designing specific compounds.
Brief overview
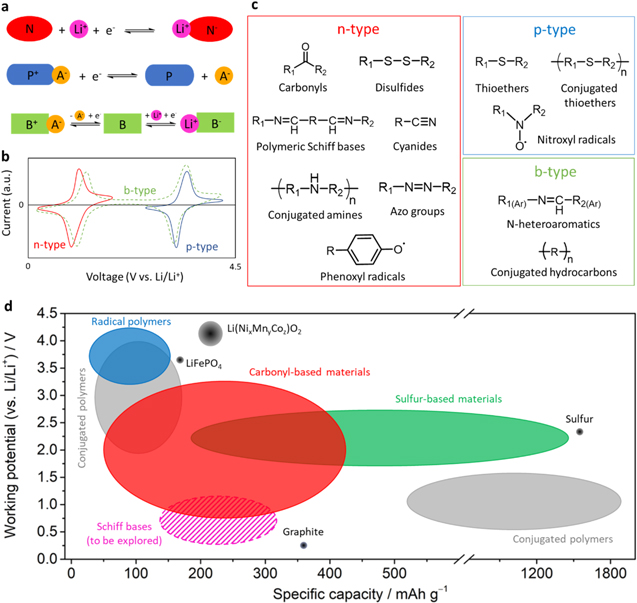
Organic electrode materials can be classified into three categories with respect to their redox reactions (Figs. 5a and 5b). For p-type (P+/P) materials, the neutral site (P) can be electrochemically oxidized to become positively charged (P+). In this state, the anion of the electrolyte (e.g., PF6−, TFSI−, BF4−, ClO4−...) is necessary to compensate the created positive charge. Regarding n-type (N/N−) materials, the neutral state (N) can be reduced to form the negative form (N−). Here, it is the cation (e.g., Li+, Na+, K+, Mg2+, ...) that is needed in vicinity of those groups/molecules to compensate the negative charges. Finally, b-type (B+/B/B−) materials, also known as bipolar type can be electrochemically oxidized (B+) or reduced (B−). However, they are often employed as only n- or p-type depending on their most suitable electrochemical activity and stability. Several active groups or reaction mechanisms have been studied as shown in Fig. 5c and their main specific capacities are shown in Fig. 5d.
Figure 5. (a) Redox reaction of the three electroactive organic types, with A− the anion and Li+ the cation (that can be replaced by other alkali ions); (b) the representative cyclic voltammetry of these three types, inspired from Ref. 40; (c) types of organic electrode materials; (d) specific capacities of organic materials,41 with the addition of the latent improvement awaited using Schiff bases (in stripes).
Download figure:
Standard image High-resolution imageContrary to inorganic intercalation electrodes, organic electrodes are not limited by the size of ions to have an electrochemical activity. Therefore, this enables the same material to be used as active material for different battery technologies (Li-, Na-, K-, Mg-ion batteries) with different electrolytes (organic or aqueous) depending on the stability of the organic material.
The topic of active organic materials is currently deeply reviewed,40–43 with some of these papers focusing on the practicality of organic batteries.44,45 Each class of organic materials has advantages and drawbacks. To sum up, conjugated polymers have a high electronic conductivity but have sloping voltage profiles and become unstable upon cycling due to doping changes while carbonyl based compounds have high capacity and fast kinetics, but can have solubility issues in electrolyte, and display a low electronic conductivity. Sulfur-based compounds have a high capacity but have low kinetics, low electronic conductivity, and solubility issues. Radical compounds have very fast kinetics, a well-defined redox potential, but often have low capacities, low conductivity, high self-discharge, and solubility issues. Finally, the others (e.g., Schiff bases and azo compounds) often have high capacity and fast kinetics, but have low ionic conductivities and solubility issues. Furthermore, since computational studies of organic materials are becoming extremely efficient, deeper understanding of mechanism and rationalized innovation/design at the structural level can be expected.46 So, commercialization of organic material seems viable, and will be soon mainly limited only by advancements in chemical industries for reaching a sustainable production.44
The next part will describe the main events in organic batteries out of which the work of Michel Armand influenced this innovative and probably near future key technology.
Materials of current interest
Organic-based electrodes development is as old as lithium batteries. The first report of this type of electrodes can be traced back to the late 1960s, with the use of dichloroisocyanuric acid as cathode material for primary battery.47 Later, several small organic molecules were tested as cathode material (e.g., quinones, dianhydrides, and phthalocyanines). The key improvement was the emergence of conductive polymers that initiated an intensive investigation of organics as electroactive materials from 1980s to 1990s (e.g., poly(acetylenes), polypyrroles, polythiophenes, polyanilines).48 These polymers can reach a high specific capacity by doping. It is during this period that the first paper of Michel Armand emerged on this topic, with the utilization of poly(decaviologen) as anode electrode when he was at the University of Grenoble.49
However, since these conductive polymers display lower stability than inorganic electrodes, research on these materials slowed down, in spite of several contributions being made on organosulfides. Another noticeable contribution of M. Armand during that time was done when he was a professor at the University of Montreal, with patents filed on polyquinonic ionic compounds.50,51
The year 2000 can be traced as the reinvigoration and intense development of organic electrodes. The discovery of new organic electroactive materials at this date, such as nitroxyl radical polymers and conjugated carbonyl compounds, gave an exponential growing interest to this topic.52 At LRCS, University of Picardie Jules Verne, Amiens, M. Armand with J.-M. Tarascon and P. Poizot have contributed at large to the popularity of organic batteries, by being one of the first to point out the capability and necessity of organic electrodes.53,54 Also, they revealed a new class of material, the organic salts, and some conjugated carbonyl compounds that can easily be extracted from biomass.55 To sum up, these metal organic salts are based on dicarboxylic acids, hydroxyl quinones, and imides, that solve the solubility problem of small organic molecules.56–58 Furthermore their organic electrode using Li-terephthalate became a reference for organic anode material, with a redox potential of 0.8 V vs Li/Li+, capacity of 300 mAh g−1, and moderate capacity retention (78% after 50 cycles).59
Finally, a novel useful redox active entity, the Schiff base, was revelated for sodium ion batteries by M. Armand and co-workers at CIC Energigune.60–62 These promising compounds still, to date, need to be explored. Several other contributions were made by him on polyimide-polyethers,63–65 organosulfur polymers,66 poly(quinones-amine),67 radical polymers,68 and organic-nitrogen compounds69 (work at IMN in Nantes).
Polymer Electrolytes
Lithium-ion batteries have reached their threshold limits of 300 Wh kg−1 that falls short of meeting the current energy storage requirements. Alternative battery systems employing Li metal such as Li metal polymer batteries, Li/S batteries, and Li-O2 batteries are currently probed. Solid-state batteries using lithium metal (SSLB) are of high interest due to their potential benefits in gravimetric and volumetric energy density and safety when compared to traditional liquid electrolyte-based systems. The ever-growing need for designing flexible energy storage devices for use in mobile electronics has kindled the growth of solid-state battery market.
Key developments
Ionic conductivity was first observed by P.V. Wright and co-workers70,71 in a poly(ethylene) oxide PEO complex with sodium/potassium thiocyanates and sodium iodide. The solubility of PEO was found to be strongly influenced by certain metal salts and the conductivities observed in polymers were attributed to the electronic mobility. In 1978, Michel Armand72,73 proposed the first PEO/Li+ dry solid polymer electrolyte (SPE) system (∼10−4 s cm−1 at 40 °C–60 °C) for lithium batteries. His pioneering work led to a major breakthrough in solid-state rechargeable batteries that led to widespread attention. The ionic conduction in PEO-based electrolytes occurs through the dissociation of Li ions from the counter ion and coordinating to the electron-donor groups present in the polymer host followed by hopping from one coordinating site (usually composed of more than 3 electron donor groups) to another. Segmental motion of the polymer chain facilitates ion hopping (Fig. 6a) along the glassy/amorphous regions of the SPE, or by the ion cluster-assisted hopping mechanism, in which temporary re-association with the counter ion occurs before being re-solvated by electron donor groups in the polymer.
Figure 6. (a) Li-ion conduction mechanism in PEO polymer electrolyte; (b) Li salts with bulky anions; (c) fluorine-free Li salt.74
Download figure:
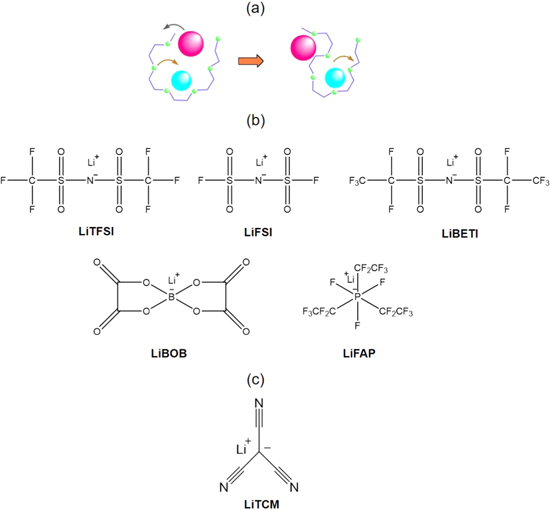
Standard image High-resolution imageIt was known by the mid-1980s that the conduction mechanism in PEO polymer electrolytes was mainly taking place in the amorphous regions of the polymers. Efforts were made to reduce the crystallinity of the polymers either by attaching PEO strands to polymer backbone or to form co-polymer with the ether strands thereby considerably reducing the crystallinity and were termed as second-generation electrolytes.75 Some of the co-polymerization techniques tested involved introducing PPO (polypropylene units (10%–20%)) in order to reduce the crystallinity as well as grafting polyether to polysiloxane and polyphosphazene backbone. Although co-polymerization improved the amorphous nature of the polymer, when used as polymer hosts with lithium salts, the transport properties were not appealing enough to be used for practical lithium batteries.76 The conductivity of the polymer electrolytes also depends on the lithium salts employed. Simple salts such as LiCl do not provide good conductivity thereby leading to search for salts with well-delocalized negative charge and basicity. LiClO4 lithium salt introduced earlier was a safety issue due its oxidative capabilities and LiPF6 due to the formation of HF gas. For the first time, Michel Armand synthesized and suggested the use of imide base salt LiTFSI (lithium bis (trifluoromethane sulfonyl) imide)77 due to its high conductivity in polymer electrolytes as a result of the salt acting as an effective plasticizer. This was attributed to the bulky anions. Consequently, new salts with bulkier anions were proposed (Fig. 6b) to have a higher dissociation property and improve the plasticizing capability of the salt. Another recent improvement in the development of salts by Michel Armand includes the synthesis of a fluorine-free salt (Fig. 6c) with a highly performing salt anion, tricyanomethanide [C(CN)3– or TCM–].74 LiTCM/PEO, as a designer polymer electrolyte, was investigated and detailed in all solid-state lithium sulfur batteries. The salt anion was shown to form a robust, highly covering, and ionically conductive passivation layer in comparison to the state-of-the-art fluorinated LiTFSI-based electrolytes. This was an effort to make non-fluorinated cost-effective salts as fluorine chemistry is expensive. Moreover, fluorinated salts are denser materials that affects the volumetric capacity as well as results in the formation of a thick SEI that could be detrimental towards the performance of batteries. The development of Li metal polymer battery systems has led to the commercialization of this technology by Bollore group (Blue car® and Blue Bus®) since 2011.
Single-ion conducting polymer electrolytes
Currently all-solid-state lithium batteries are attracting more attention, either based on conventional solid polymer electrolyte (SPE) formed by simple dissolution of lithium salts in a polymer matrix or based on the single Li-ion conductors (SLIC).78–82 The suppression of volatile and flammable organic solvents was a main driver of such development towards safer lithium batteries. In addition, the shape versatility and mechanical stability comfort the observed increase on SPE development. Particularly, the polymeric SLICs family has showed remarkable progress in the last decade where many strategies have been reported.79,80 Polymeric SLICs were designed to overcome key drawbacks associated with dual-ion conducting SPEs, especially, their lower Li-ion transference number. The anion accumulation within the anode side in the conventional SPEs is assumed to lead to polarization. Thus, poor performances (e.g., voltage losses, high internal impedance and parasitic reactions) due to the generated concentration gradient can be avoided by designing SLICs with transference number approaching unity.83 Furthermore, it is predicted that the use of SLICs can suppress the Li dendrites growth and improve the cycling performances at relatively high current.80 The necessity of developing, versatile, safe, and high-performance batteries was driving an extensive work for the development of SPEs in general and particularly polymeric SLICs.78–81,84 Since the 1980s, the elaboration of new materials was focused on the selection of the architecture, the mechanism of anions encoring, the design, and the synthetic strategy.78–80,85–89
Several strategies were investigated and were mostly focused on the immobilization of the anions within polymeric backbone or within inorganic matrix. M. Armand et al. reported on the functionalization of SiO2 and Al2O3 nanoparticles by effective immobilization of organic moieties bearing Li salt (Fig. 7a).90 The prepared Li-ion conducting inorganic–organic hybrid electrolytes showed practical conductivities at relatively high temperatures and a dependence on the particle size. Furthermore, the incorporation of soft polymer segment of polyethylene glycol (PEG) into the inorganic matrix was reported to improve even more the ionic conductivity. In other words, the co-grafting of the organic moieties bearing the Li-ion and the polymer segment (PEG) helped the solvating and desolvating of the Li ions.90
Figure 7. Structure of different SLICs; (a) co-grafted with PEG on SiO2 and Al2O3 nanoparticles90; (b) structure of SLIC triblock copolymer88; (c), (d) homo and copolymer of LiPSTFSI87; (e) homopolymer of LiPSsTFSI86; (f) quasi SLIC from biopolymer backbone modification.91
Download figure:
Standard image High-resolution imageOther approaches using polymer backbone to prepare SLICs were reported and can be obtained via two strategies. The first method consisted in the synthesis of lithium salt monomer and their subsequent polymerization.86–88,92 The second one consisted on the chemical modification of commercially available and existing polymer backbones.91,93
Using the first approach, Armand et al. presented the first example of triblock copolymer consisting of polystyrene block bearing a TFSI group and PEO block (Fig. 7b). The PSTFSILi-PEO-PSTFSILi copolymer were highlighted as quasi SLICs, with transport number values superior to 0.85 and an ionic conductivity value of 1.3 × 10−5 S cm−1 measured at 60 °C.
The prepared SLICs exhibited good mechanical properties and extended electrochemical stability window up to 5 V (vs Li/Li+). Moreover, cyclability and performances of these materials were evaluated using lithium-metal battery prototypes showing impressive results. This approach promoted the development of more SLICs based on the polymerization of factionalized monomers. Armand et al. also reported on SLIC obtained by the polymerization of lithium (4-styrenesulfonyl) (trifluoromethanesulfonyl)imide (LiSTFSI) to obtain the poly(4-styrenesulfonyl) (trifluoromethanesulfonyl)imide (LiPSTFSI)89 as shown in Fig. 7c. The LiPSTFSI/PEO blend showed relatively high ionic conductivity (∼10−5 S cm−1) at 70 °C. To further improve the ionic conductivity of the LiPSTFSI polysalt, another SLIC was developed by copolymerization of the LiPSTFSI and the methoxy-polyethylene glycol acrylate (MPEGA) (Fig. 7d).87 The combination of the flexible/delocalized nature of the –SO2–N–SO2–CF3 structure in the polysalt and the flexible ethylene oxide (EO) oligomers were exploited in the designed SLICs. Hence, an improvement of the ionic conductivity in the temperature ranges of RT up to 60 °C was highlighted.
To even more exploit the concept of flexibility and delocalization observed in the case of the LiPSTFSI, Armand et al. have designed a novel SLIC thermally stable up to 300 °C and showing a high transference number when blended with PEO (0.91) (Fig. 7d).86 The prepared lithium polysalt was based on lithium-poly[(4-styrenesulfonyl)(trifluoromethyl(S-trifluoromethylsulfonylimino)sulfonyl)imide] (LiPSsTFSI). The super-delocalized polyanion displayed a low glass-transition temperature and exhibited a high Li-ion conductivity (1.35 × 10−4 S cm−1 at 90 °C) when blended with PEO.
As a second strategy, the functionalization of already existing and commercially available polymers was also explored. Recently, Michel Armand's team presented SLICs polymers based on blends of PEO with respectively functionalized poly(acrylic acid) and ethyl cellulose (Fig. 6e).91,93 Both polymers were functionalized by anion of lithium salt LiTFSI, reaching conductivities of 1.77 × 10−5 S cm−1 measured at 80 °C and 5.2 × 10−5 S cm−1 measured at 70 °C for PEO/PA-LiTFSI and PEO/ethylcellulose-LiTFSI SPEs, respectively. It was clearly emphasized that the design of the polysalt-like architecture needs to consider the elasticity of the material. Indeed, the incorporation of spacer with low energy of conformational changes within the copolymer backbone and the pending salt part is a key parameter to ensure a better dissociation rate. Still one of the major challenges facing the development of SLICs is the achievement of practical ionic conductivities (10−4 s cm−1) at RT. Thus, further work should be directed toward the improvement of the performance of these materials and their electrochemical stability at lower temperatures and at high charge/discharge rates.
Concluding Remarks
Reducing carbon emission and powering the planet with low cost energy is a prime challenge for the 21st century. Electrification of the transportation sector along with efficient energy storage systems for clean energy harvesting is currently a vital point. Major developments in battery materials during the 1970s were considered as the Holy Grail that led to the development of efficient energy storage systems. Although the developed materials improve the performance of the batteries, the safety requirements of energy storage devices, especially Li-ion batteries, were met with the development of LiFePO4 cathode material by John B. Goodenough and solid-state polymer batteries by Michel Armand. Solid-state batteries can go a long way in realizing the goals of storage devices in terms of safety, design flexibility, and high energy density (Li metal polymer, Li–S, Li-air). Transition metal oxide-based electrodes are promising, but optimization is still required to bring about a safe system due to the partial pressure of the oxygen that grow exponentially with voltage. Organic electrodes are viewed as a viable alternative that has been under intense research earlier, but some have lost their glory due to their intrinsic problems leading to dissolution of the electrodes. Recent developments by Armand on Schiff bases seem to be promising and research in this direction seems to be quite vital.
The first cycle irreversibility that is observed in batteries, not limited to carbon or conversion-based electrodes, is an immense issue that has been neglected. The concept of sacrificial salts proposed by Armand is an efficient strategy that could be adopted in any alkali metal battery systems and tuned according to the cathode materials employed. The family of sacrificial salts are cost-effective, facile, and more efficient than any other methods of metallization, and more focus needs to be diverted towards this strategy. The current performance developments in Li air batteries could be more rapid with researchers getting to know more about the family of boron complex electrolyte additives that results in increased dissolution of Li2O and of Li2O2 thereby facilitating efficient recharging capabilities.34 These additives are not limited only to Li air batteries but also towards Li-ion batteries. The tunable quality of the additives makes it adaptable for systems with new lithium salts regardless of the thick SEI formed with bulkier fluorinated anions.
The development of imide-based salts by Armand have led to considerable improvement in polymer electrolytes and ionic liquids. At the same time, development of non-fluorinated salts and research focus on improving their ionic conductivity is quite important that needs further attention. Current polymer-in-salt systems are impressive in terms of performance but in practice quite expensive. The salts form a major component of a polymer electrolyte and non-fluorinated salts could be a boon in disguise due to the fact that fluorine chemistry is time consuming and expensive. Dendrite formation due to concentration gradients in Li metal battery systems has been a hindering factor for their development. Single-ion conducting polymer electrolytes as shown by Armand are viable for solid-state polymer electrolytes and polymer hybrid materials, and research towards improving their ionic conductivity should be considered as prima facie research. Another important field of research not included in this review is ionic liquids that definitely add up to the development of electrolytes in terms of improving electrochemical stability of the polymer electrolytes. To conclude, future improvements in lithium battery technology holds promises with polymer electrolytes or polymer hybrids using ceramic nano-particles and ionic liquids with Li metal anodes.
The research on materials for lithium batteries has seen various stages of improvements that have only been possible due to the contribution of various eminent scientists from all over the world. Nevertheless, the key findings from Michel Armand seem to form a major portion of the developments. A visionary like Michel Armand is an asset to the scientific community, who has inspired young minds throughout his career and implanting new ideas. We hope that the key developments that have been mentioned in this review on the works carried out by Michel Armand would inspire young talents from the scientific community to proceed further on the research directions mentioned. These developments will enable us to reach our goal of clean energy production and storage that in turn is crucial for our planet to mitigate the existing global climate issues.
Acknowledgments
D. Shanmukaraj, P. Ranque, and T. Rojo gratefully acknowledge the support from CIC Energigune. Alava, Spain; S. Grugeon and S. Laruelle thank the support from LRCS, University of Picardie Jules Verne, Amiens, France; P. Poizot and D. Guyomard gratefully thank the support from the Institut des Materiaux Jean Rouxel (IMN), the Centre de la Recherche Scientifique (CNRS) and the University of Nantes, France. The permission from the authors for generous reproduction of figures is gratefully acknowledged.