Abstract
Cathode material with high nickel content is a promising candidate for the future generation of Li-ion batteries (LIBs). However, severe structural degradation during cycling limits its practical use, especially for electric vehicles. Herein, AlPO4 nanoparticles were synthesized and then coated onto the surface of a high-nickel layer-structured cathode via a dry coating method. The AlPO4 nanoparticles coating significantly improved the cycling stability from 69.2% to over 80% capacity retention after 140 cycles. Furthermore, the structure and chemical composition of the AlPO4-coated cathode was investigated by XRD, SEM, XPS, and STEM. Compared with the non-coated cathode, we revealed a dual protective mechanism for enhanced cycling stability, where Al doping and Li3PO4 coating play synergistic roles in protecting cathode material through long-term cycling. This work demonstrates a facile and environmentally friendly approach toward improving the performance of high-nickel LIB cathodes, which can be easily scaled up for industrial applications.
Export citation and abstract BibTeX RIS
The urgent demand for power batteries accelerates the revolution of state-of-the-art cathode materials toward low cost and high performance, departing from commercial layer-structured LiCoO2 due to their high cost, toxicity, and mediocre performance. 1–4 The high-nickel layered cathode materials with lower cobalt content are promising alternatives because of their high specific capacity and high nickel abundance. 5,6 However, high-nickel cathode materials also face severe problems, typically, such as poor structural stability during cycling. First, the high chemical reactivity of Ni3+/2+ and similar ionic radii of Li+/Ni2+ can induce the cation migration. 7 This migration behavior, so-called cation mixing, causes an irreversible structure change to spinel or further to rock-salt structure, especially on the surface, and hinders Li-ion diffusion. 8–10 In addition, the severe volume expansion during delithiation also causes repulsion between primary particles and results in micro- and nano-cracking within secondary particles of the cathode materials, deteriorating the high-nickel cathode materials performance. 11,12 These cracks expose more surface to the electrolyte and act as catalytic points for electrolyte decomposition, which blocks the intraparticle electrical contact between primary particles. 13,14 Furthermore, the generation of acidic compounds from the trace water leads to transition metals dissolution and segregation. The crossover effect is another issue for capacity fading. 15 Therefore, it is necessary to find an approach to improve high-nickel cathode materials, where the layered structure is stabilized to avoid cation mixing and cracking and the surface is protected from undesirable parasitic reactions with electrolytes.
The coating is an effective approach to enhance the cyclability of layered cathode materials. 11 Among coatings materials, AlPO4 is a representative candidate due to its lower cost, similar chemical stability, and better ionic conductivity compared to Al2O3 and AlF3, 16 which has been used to improve the thermal stability of LiCoO2. 16,17 Intensive research about this coating effect has been carried out on the family of layer-structured cathodes. AlPO4 has been coated onto the cathode surface using various deposition methods, e.g., wet coating, 18–20 atomic layer deposition, 21 etc. As a result, the AlPO4 coating improves the thermal stability and the capacity retention of layer-structured cathode materials. This is attributed to the strong bonding between (PO4)3− polyanion and Al3+ cation, enhancing resistance to the reaction with electrolyte. 22,23
Hitherto, AlPO4 coating had been barely reported and applied to high-nickel layer-structured cathode materials. In this paper, different from conventional AlPO4 coating methods on layer-structured cathode materials, AlPO4 coating was introduced via a nanoparticle dry coating approach to the cathode precursor materials. In this manner, upon calcination of the AlPO4 coated precursor with LiOH, the high-nickel layered cathode materials were doped by Al and its surface was protected by as-formed Li3PO4 coating, rather than common AlPO4 coating on the cathode materials. The dual protective mechanism of Al doping and Li3PO4 coating can improve the cycling performance and structural stability of the high-nickel cathode material, as demonstrated in Li(Ni0.92Co0.04Mn0.04)O2 (NCM9244). With detailed characterizations of the cathode materials using Scanning Electron Microscopy (SEM), X-ray Photoelectron Spectroscopy (XPS), high-resolution Scanning Transmission Electron Microscopy (STEM), and Energy-Dispersive X-ray Spectroscopy (EDS), we found that deep doping of Al3+ into the bulk structure in primary particles slows down surface reconstruction from layered structure to spinel/rock-salt structures and thus improves the reversibility of the layered structure upon cycling, while the rigid phosphate surface layer can protect the cathode surface from the electrolyte and its decomposed products. Collectively, the dual protective approach enables the high nickel NCM9244 cathode materials to cycle with reduced microstructure crack of its spherical particles and decreased parasitic reaction with electrolyte, achieving improved cycling stability than the non-coated cathode materials. This one-step, dual protective strategy has great potential to be applied in large-scale production for high energy and long-cycle-life high-nickel cathode materials.
Experimental
Materials synthesis
AlPO4 nanoparticles were synthesized by an aqueous precipitation method. AlCl3·6H2O and (NH4)H2PO4 were dissolved at the same concentration of 0.05 M into deionization (DI) water and stirred for 30 min. Diluted ammonia solution (1.5 wt%) was slowly added under vigorous stirring. Gel-like precipitation product was washed with DI water, collected by centrifuging, and dried under vacuum at 40 °C for 24 h.
High-nickel cathode precursor Ni0.92Co0.04Mn0.04(OH)2 synthesized by the co-precipitation method was used as the starting material. Bare cathode material LiNi0.92Co0.04Mn0.04O2 was synthesized by mixing the high-nickel precursor with LiOH·H2O with a molar ratio of transition metal: Li = 1:1.05, followed by calcination under oxygen flow at 480 °C for 5 h, then at 740 °C for 15 h. The AlPO4-coated NCM9244 was prepared by mixing the precursor, AlPO4, and LiOH·H2O together for calcination. The weight percentage of AlPO4 to the cathode precursor was selected to be 0.5, 1 to 2 wt%. The molar ratio of transition metal to Li and the calcination procedure are the same as for the bare sample. The calcined, AlPO4-coated NCM9244 cathode materials with 0.5 wt%, 1 wt%, and 2 wt% AlPO4 were designated as 0.5% APO, 1% APO, and 2% APO, respectively.
Material characterization
The crystalline structures were analyzed using X-ray Diffraction (XRD) performed on Rigaku MiniFlex II Desktop X-ray Diffractometer, Japan. The XRD data were acquired using Cu Kα radiation source at a scanning range of 2θ from 10° to 80° with 0.02°/step. Particle morphologies of as-synthesized samples were studied by SEM (Apreo 2, Thermo Fisher Scientific) with a secondary electron detector. The particle size was measured by a volumetric laser diffraction machine (Mastersizer 3000, Malvern). Cross-sections of the electrodes were performed using ion beam milling (Leica EM TIC 3X), followed by imaging under SEM. We conducted XPS (XPS Versaprobe II, PHI) to determine the surface chemical composition on bare and coated electrodes.
Representative samples were selected for the STEM study and the STEM-EDS analysis along the cross-sectional direction. The STEM lamella specimens were prepared by the Focused Ion Beam (FIB) technique. FEI Helios Hydra UX FIB-SEM was used for lift-out using the Xe ion source operated at 30 kV, and FEI Helios 600 FIB-SEM was used for fine thinning by the Ga ion source at 30 kV to 2 kV. The STEM studies were performed on a transmission electron microscope (Themis Z, ThermoFisher) operated at 300 kV. The high-angle annular dark-field (HAADF) images were collected in the angle range of 65–200 mrad. The STEM-EDS measurements were performed on the transmission electron microscope fitted with Super-X spectrometer with 4 detectors and 0.8 srad solid angle.
Electrochemical test
Cathode materials were mixed with carbon additives (Super C45, MTI Corporation) and polyvinylidene binder (PVdF, Sigma Aldrich) at a weight ratio of 8:1:1 and cast onto aluminum foil. The active material loading is ∼2.5 mg cm−2. Electrodes were punched and assembled in CR2016 coin cells with Li foil, Celgard 2320, and LP71 as the counter electrode, separator, and electrolyte, respectively. Charge-discharge cycling and electrochemical behavior of cathode materials were measured on a CT2001A Battery tester, Landt Instruments. Cells were charged and discharged between voltage windows of 2.7–4.3 V at a 0.1C rate for 3 cycles, followed by a 1C rate for the rest cycles of 50-cycled and 200-cycled samples.
Results and Discussion
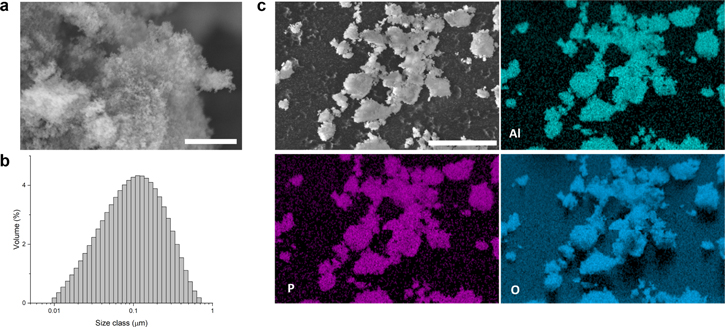
As-synthesized AlPO4 nanoparticles were characterized using SEM, XRD, and laser diffraction techniques to illustrate their morphology and particle distribution. As shown in Fig. 1a, as-synthesized AlPO4 comprises clusters of loosely aggregated nanoparticles with a measurable particle size of 50 nm. XRD patterns show a broad reflection peak, indicating the amorphous feature of the nanoparticles (Fig. S1 (available online at stacks.iop.org/JES/169/050523/mmedia) in Supporting Information). According to the laser diffraction technique (Fig. 1b), the volume-weighted size distribution exhibits a bell-shaped curve with a Dx(10) value of 33 nm and a Dx(50) value of 139 nm. The SEM-EDS mapping indicates a uniform distribution of the Al, P, and O elements in the AlPO4 nanoparticles (Figs. 1c and S2).
Figure 1. Characterization of as-synthesized AlPO4 particles: (a) SEM image, the scale bar is 1 μm. (b) Volume-weighted size distribution measured by laser diffraction technique. (c) SEM-EDS mapping, the scale bar is 25 μm.
Download figure:
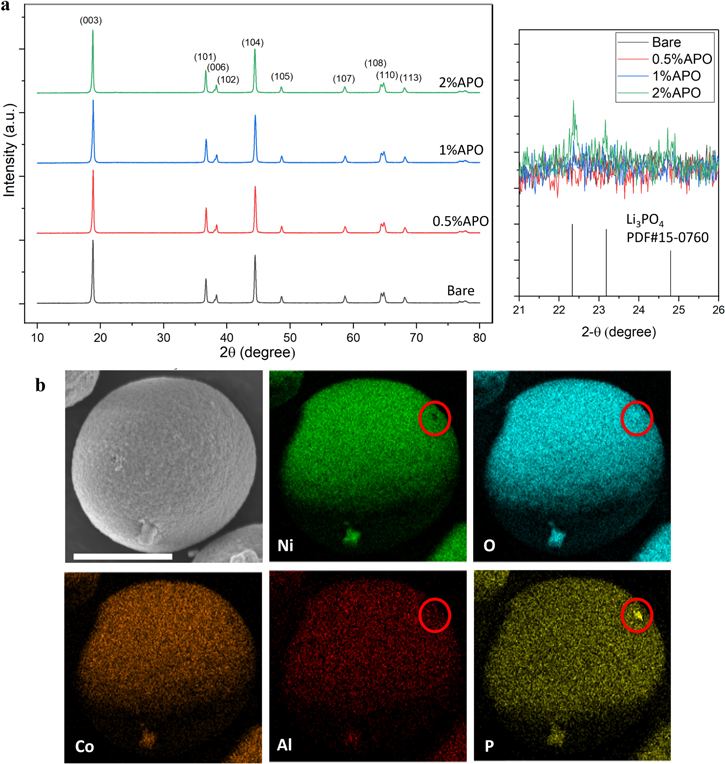
Standard image High-resolution imageThe AlPO4 nanoparticles were then used to coat the NCM9244 precursor via a dry mixing procedure, followed by calcination to obtain the AlPO4-coated NCM9244 cathodes. XRD was performed to investigate the crystal structure of as-synthesized NCM9244 cathode materials, including the bare cathode, 0.5% APO, 1% APO, and 2% APO (Fig. 2a). All the peaks from these samples are well-indexed and attributed to the hexagonal α-NaFeO2 structure in  space group. Peaks of impurity (enlarged area of Fig. 2a) are only detected on the XRD pattern for the 2% APO sample, which correspond to the Li3PO4 phase (PDF#15–0760), suggesting excessive AlPO4 coating material used in the 2% APO sample. The clear peak splittings of (006)/(102) and (108)/(110) for all four cathode materials imply a separation of Li and transition metal slab layers and a stable phase of the layered structure formed. The intensity ratio between (003) and (104) peaks, a value commonly used to indicate the cation mixing degree of the layered structure,
7
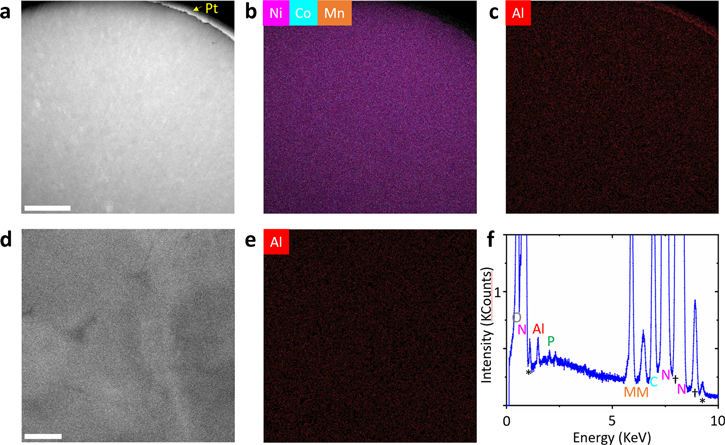
slightly increases from 1.30 for the bare NCM9244 to 1.34, 1.34, and 1.36 for the 0.5% APO, 1% APO, and 2% APO, respectively, suggesting an improvement in cation distribution in their own slabs and less cation mixing occurrence in the coated samples. The 1% APO cathode material was further investigated with in-depth characterization. The 1% APO shows spherical morphology (Fig. 2b) and the SEM-EDS mapping demonstrates a uniform distribution of Al and P elements at the surface of the spherical cathode particles, indicating the uniform distribution of AlPO4 nanoparticles onto the NCM9244 precursor after the dry coating. Interestingly, after calcination, there exists a small spot of stronger signal on the P and O mapping, absent on the Ni and Al mapping (see red circles). This observation implies that the (PO4)3-, possibly Li3PO4 rather than AlPO4, stays on the calcined NCM9244 surface. Al distribution inside the 1% APO cathode particles is further illuminated by STEM-HAADF imaging and STEM-EDS mapping. Figure 3c shows the Al STEM-EDS mapping of a cross-sectional 1% APO secondary particle, and the Al mapping is referred to the STEM-HAADF image (Fig. 3a). The Ni/Co/Mn STEM-EDS mapping (Fig. 3b) was collected simultaneously in the same area. Since the mapping area is large enough to cover both the surface and center part of the secondary particle, Al is found randomly distributed over the particle, which evidences the incorporation of Al into the entire secondary particle. One may note that the comparatively stronger STEM-EDS Al signal at the secondary particle surface in Fig. 3c, this area matches with the Pt cap layer, which was used for sample protection during FIB in Fig. 3a, so that the stronger STEM-EDS Al signal at the surface is assigned to artifacts caused by the heavy element Pt in the cap layer. The X-rays emitted from Pt propagate in all directions and can further excite different areas other than the area directly hit by the electron beam. Such artifacts are also be found in the STEM-EDS Ni/Co/Mn mapping in Fig. 3b, however, the artifacts are more pronounced for the light element Al. On the other hand, due to the overlapping of P-Kα edge and Pt-M edge, the existence of phosphate on the secondary particle surface is hard to determine. Hence, no significant AlPO4 residual is found at the secondary particle surface. A further inspection of the secondary particle center (Figs. 3d–3f) shows Al is indeed incorporated into the secondary particle center and randomly distributed. Higher magnification of STEM-EDS mapping performed on the edge of a primary particle shows that P is only found at the primary particle surface, as shown in Fig. S5, not in the bulk structure. In contrast, Al distributes randomly inside the particle with the negligible signal on the primary particle edge. Based on the STEM and SEM analysis, the 1% APO cathode particles, prepared by the AlPO4 dry coating approach, incorporate Al into the bulk and form a phosphate coating on the primary particle surface.
space group. Peaks of impurity (enlarged area of Fig. 2a) are only detected on the XRD pattern for the 2% APO sample, which correspond to the Li3PO4 phase (PDF#15–0760), suggesting excessive AlPO4 coating material used in the 2% APO sample. The clear peak splittings of (006)/(102) and (108)/(110) for all four cathode materials imply a separation of Li and transition metal slab layers and a stable phase of the layered structure formed. The intensity ratio between (003) and (104) peaks, a value commonly used to indicate the cation mixing degree of the layered structure,
7
slightly increases from 1.30 for the bare NCM9244 to 1.34, 1.34, and 1.36 for the 0.5% APO, 1% APO, and 2% APO, respectively, suggesting an improvement in cation distribution in their own slabs and less cation mixing occurrence in the coated samples. The 1% APO cathode material was further investigated with in-depth characterization. The 1% APO shows spherical morphology (Fig. 2b) and the SEM-EDS mapping demonstrates a uniform distribution of Al and P elements at the surface of the spherical cathode particles, indicating the uniform distribution of AlPO4 nanoparticles onto the NCM9244 precursor after the dry coating. Interestingly, after calcination, there exists a small spot of stronger signal on the P and O mapping, absent on the Ni and Al mapping (see red circles). This observation implies that the (PO4)3-, possibly Li3PO4 rather than AlPO4, stays on the calcined NCM9244 surface. Al distribution inside the 1% APO cathode particles is further illuminated by STEM-HAADF imaging and STEM-EDS mapping. Figure 3c shows the Al STEM-EDS mapping of a cross-sectional 1% APO secondary particle, and the Al mapping is referred to the STEM-HAADF image (Fig. 3a). The Ni/Co/Mn STEM-EDS mapping (Fig. 3b) was collected simultaneously in the same area. Since the mapping area is large enough to cover both the surface and center part of the secondary particle, Al is found randomly distributed over the particle, which evidences the incorporation of Al into the entire secondary particle. One may note that the comparatively stronger STEM-EDS Al signal at the secondary particle surface in Fig. 3c, this area matches with the Pt cap layer, which was used for sample protection during FIB in Fig. 3a, so that the stronger STEM-EDS Al signal at the surface is assigned to artifacts caused by the heavy element Pt in the cap layer. The X-rays emitted from Pt propagate in all directions and can further excite different areas other than the area directly hit by the electron beam. Such artifacts are also be found in the STEM-EDS Ni/Co/Mn mapping in Fig. 3b, however, the artifacts are more pronounced for the light element Al. On the other hand, due to the overlapping of P-Kα edge and Pt-M edge, the existence of phosphate on the secondary particle surface is hard to determine. Hence, no significant AlPO4 residual is found at the secondary particle surface. A further inspection of the secondary particle center (Figs. 3d–3f) shows Al is indeed incorporated into the secondary particle center and randomly distributed. Higher magnification of STEM-EDS mapping performed on the edge of a primary particle shows that P is only found at the primary particle surface, as shown in Fig. S5, not in the bulk structure. In contrast, Al distributes randomly inside the particle with the negligible signal on the primary particle edge. Based on the STEM and SEM analysis, the 1% APO cathode particles, prepared by the AlPO4 dry coating approach, incorporate Al into the bulk and form a phosphate coating on the primary particle surface.
Figure 2. (a) XRD patterns of bare, 0.5% APO, 1% APO and 2% APO cathode materials. The area where diffraction peaks of Li3PO4 (PDF#15-0760) are detected is enlarged. (b) SEM-EDS mapping of 1% APO cathode materials. The scale bar is 10 μm.
Download figure:
Standard image High-resolution imageFigure 3. (a) Cross-sectional STEM-HAADF image of the 1% APO secondary particle, the Pt cap layer for sample preparation on the spherical particle surface is marked, the scale bar corresponds to 2 μm; (b), (c) STEM-EDS mapping on the same area of (a), the scale bar in (a) applies to (b) and (c); (d) cross-sectional STEM-HAADF image of the secondary particle center part, the scale bar corresponds to 50 nm, together with the corresponding (e) STEM-EDS Al mapping and (f) STEM-EDS spectrum of the 1% APO coated cathode, the EDS edges are identified as the key elements Al, P, Ni(N), Co(C), Mn(M) and O († and * correspond to artifacts of Cu and Ga, respectively, due to the TEM specimen grid and sample preparation by FIB).
Download figure:
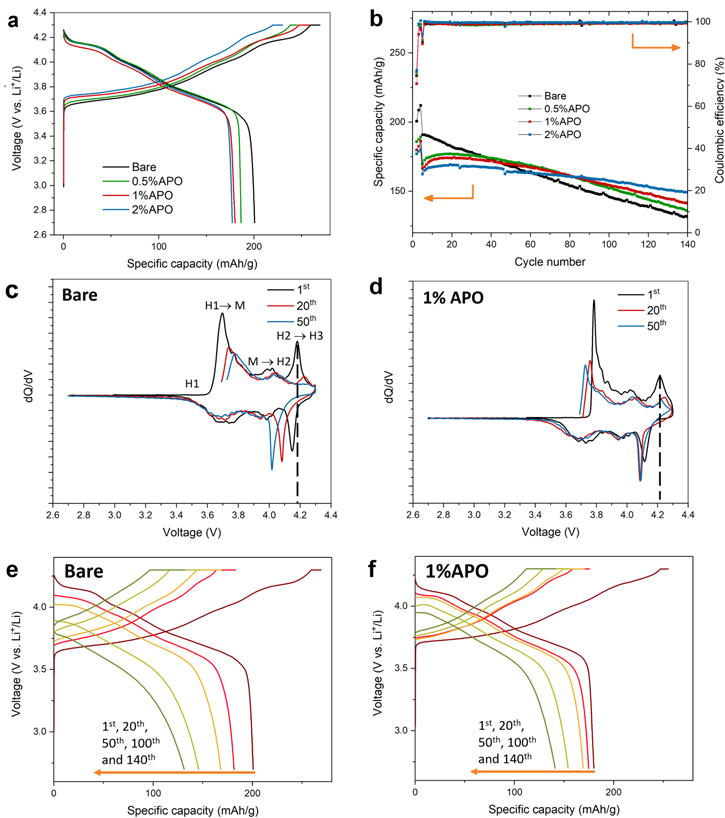
Standard image High-resolution imageElectrochemical measurements demonstrate the effects of using the AlPO4 coating on improving the cycling performance of high-nickel NCM9244 cathode materials. As shown in Fig. 4a, the coating layer increases the polarization, resulting in a capacity decay from 201.0 mAh g−1 of the bare sample to 180.2 mAh g−1 of the 0.5% APO sample and even further down to 177.5 mAh g−1 for the 2% APO sample. Nevertheless, during the long-term 140 cycles, the capacity retention of the bare electrode remains only 69.2%, while the coated cathode materials possess a significantly enhanced capacity retention rate of 80.1%, 84.8%, and 91.8% for 0.5%, 1%, and 2% APO cathode, respectively (All capacity retention values are calculated based on first discharge specific capacity at 1 C rate). Moreover, due to the elimination of water in the dry coating process, the 1% APO cathode also shows a 5% higher capacity retention than coated cathodes prepared by an aqueous wet coating method (Supporting information, Figs. S3 and S6). The dependence of specific capacity on the current rate is investigated at the current rate from 0.1C to 5C as in Fig. S7. Notably, the 1% APO cathode performs similarly to the bare sample after initial activation cycles, even at a high current rate. Moreover, after cycling at the rate of 5C, the comparatively higher recovered capacity of the 1% APO cathode indicates the improved structural stability of the coated cathode materials. In contrast, the bare sample suffers from a sudden drop in the specific capacity.
Figure 4. (a) The 1st cycle charge-discharge voltage profiles at the current rate of 0.1C of bare and coated cathode materials. (b) Discharge specific capacity over 200 cycles of bare and different coated cathode materials. (c), (d) dQ/dV curves of (c) bare and (d) 1% APO cathode material. (e), (f) Voltage profile evolution of (e) bare and (f) 1% APO cathode material.
Download figure:
Standard image High-resolution imageTo obtain a more profound understanding of the structural rearrangement of the bare NCM9244 and 1% APO cathode materials upon cycling, the dQ/dV profiles are taken at 1st, 20th and 50th cycles for bare and 1% APO cathode. Three significant oxidation peaks with peak positions from 3.6 V to 4.3 V in Fig. 4c are pinned down to phase transition from the original hexagonal layered structure (H1) to monoclinic phase (M), then to another hexagonal phase (H2) and the final hexagonal phase (H3). The drastic change in the c direction during the phase transformation from H2 to H3 has been proved to cause abrupt and anisotropic volume expansion near the end of charging. 24–29 Since cathode secondary particles are intact integration of primary particles along different orientations, the anisotropic volume expansions of primary particles over long-term cycling create mechanical stress on each other, leading to microcrack formation. The microcracks serve as channels for deleterious electrolyte infiltration and thus expedite the degradation of interior primary particles by accelerating the parasitic reactions. These increased surface area and side reactions lead to rapid capacity fading and structural degradation. 30,31 However, the existence and minor evolution of this peak on the dQ/dV profile can also be regarded as evidence of the reversibility of the whole structure. Figure 4c also shows a substantial decline in peak height and remarkable shifts in peak position for the bare samples. Noticeably, the oxidation peak at 4.2 V (H2–H3 transition) completely disappears at the 50th cycle, which demonstrates that the structural collapse is irreversible. For the 1% APO cathode, the overpotential due to the coating layer shifts the peak position from 4.18 V to 4.21 V and prolongs this phase transition. Hence, the volume expansion is slowed down and occurs less abruptly, which should be attributed to Al doping since the replacement of Ni in LiNiO2 by Al has been proved to suppress H2–H3 transition during cycling. 32 Moreover, the redox peak shifts are negligible, and there is no significant difference in peak intensity between the 20th and 50th cycle (Fig. 4d), indicating that the coating layer can stabilize the structure and improve reversibility during cycling.
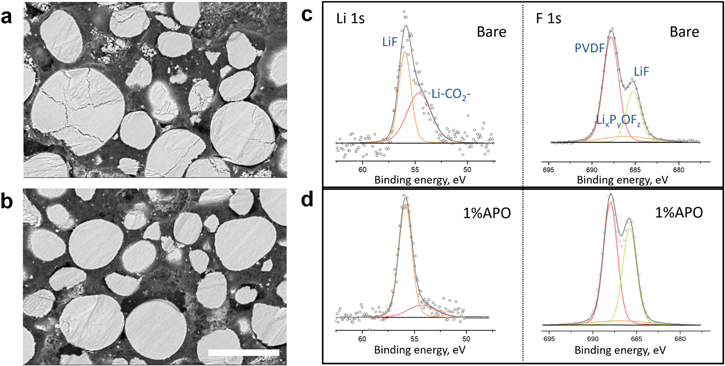
To further explore the effect of the coating layer on particle integrity, both bare and 1% APO cathodes after 200 cycles are characterized using cross-sectional SEM techniques. The secondary particles were cross-sectioned using ion milling (Figs. 5a, 5b). Cracking is observed from the center toward the surface on many bare NCM9244 particles, suggesting cracks originate from mechanical stress between primary particles due to volume expansion upon deep Li+ extraction via abrupt H2 to H3 phase transition. In contrast, for the 1% APO electrode, no noticeable cracking is observed. Such a comparison between the cycled bare NCM9244 and 1% APO cathodes shows a significant improvement in particle integrity by the AlPO4 coating, which is consistent with the observations in Figs. 4c and 4d that less abrupt change lattice parameter in the coated cathode upon cycling as discussed above.
Figure 5. (a), (b) Cross-sectional SEM images of (a) bare and (b) 1% APO cathode electrode after 200 cycles. The scale bar is 20 μm. (c), (d) XPS Li 1s and F 1s spectrum of (c) bare NCM9244 and (d) 1% APO electrodes after 200 cycles.
Download figure:
Standard image High-resolution imageXPS is applied to characterize the difference in surface components of electrodes after cycling (Figs. 5c, 5d). The Li 1 s spectrum collected on bare NCM9244 electrodes after 200 cycles manifests that the particle surface is covered by LiF (binding energy of 56.2 eV) and Li-CO2 −- containing compound (binding energy of 54.5–55.0 eV) produced by electrolyte decomposition. In contrast, LiF as the main component in cathode electrolyte interphase (CEI) is observed in Li 1s spectrum of 1% APO electrode, indicating reduced side products from parasitic reactions of electrolyte decomposition. The F 1s spectrum exhibits a similar trend: the LiF proportion on the surface dramatically increases on 1% APO cathode. These results support the Li3PO4 coating layer protecting role, 30 where the phosphate phase prohibits the etching reaction of acidic compounds from electrolyte decomposition and preserves the structural integrity inside particles. 15
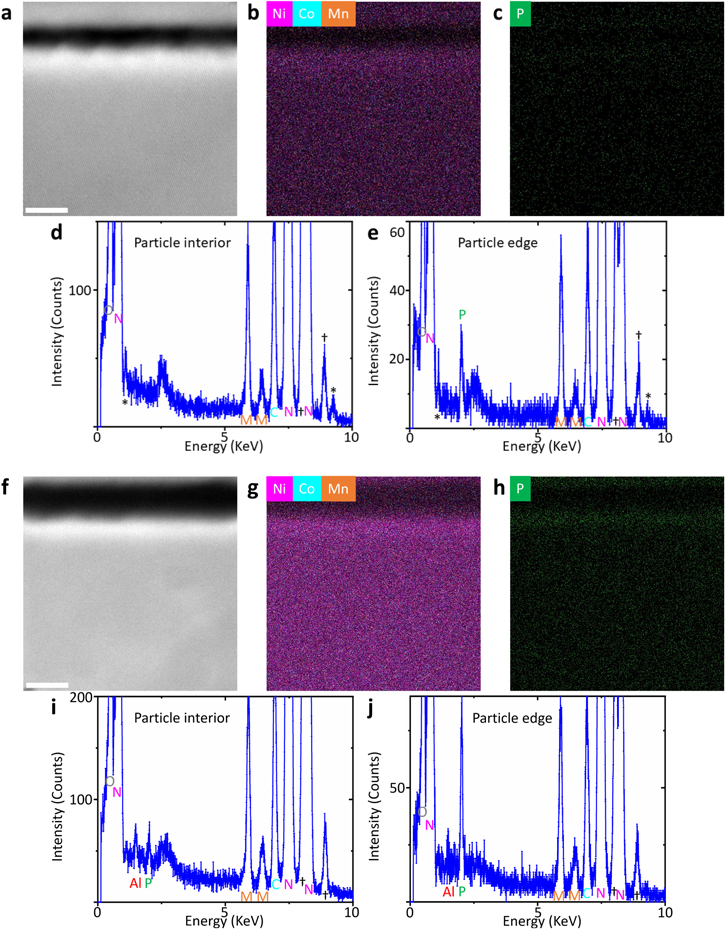
Microstructural evolution upon cycling is studied for the bare NCM9244 and 1% APO cathodes after 200 cycles by STEM-HAADF imaging and STEM-EDS analyses. Primary particle surface areas are characterized for both cycled samples of bare NCM9244 and 1% APO cathode, as shown in the STEM-HAADF images of Figs. 6a and 6f, respectively. Considering the intensity in the STEM-HAADF imaging is roughly proportional to Z2 (Z is the atomic number), the higher intensity areas in these images show where the particles are, and the lower intensity areas correspond to grain boundary areas. The comparatively higher intensity at the very surface of primary particles is due to the formation of Ni/Co/Mn-rich rock-salt reconstruction layer, which will be discussed in detail in the following part about Fig. 7. In Figs. 6b and 6g, the STEM-EDS mapping performed on the same area as in the STEM-HAADF images of both cycled samples evidences that the constituents of Ni, Co, Mn randomly distribute inside the particle but not in the grain boundary areas. Furthermore, The STEM-EDS maps in Figs. 6b and 6g also show that Ni/Co/Mn dominated layers present at the very surface of the particles, which is attributed to the formation of the reconstruction layer at the particle surface as will be discussed in Fig. 7. P is a good indicator for the interaction between the cathodes and electrolyte during cycling since P is the comparatively heavier element in the electrolyte LP71 employed in this study. Thus, STEM-EDS P mapping is also analyzed using the same data set used for the Ni/Co/Mn mapping, as shown in Figs. 6c and 6h. Even though the P signal is not so pronounced for the cycled bare NCM9244 in Fig. 6c, a careful comparison between the STEM-EDS spectra of Fig. 6d inside the particle and Fig. 6e at the particle edge apparently shows P presents only at the particle surface, which suggests a P-containing CEI formed at the particle surface after cycling. Meanwhile, the STEM-EDS spectrum from the particle interior only shows the constituents of bare NCM9244 as expected. In contrast, a much more pronounced P-containing CEI is probed by STEM-EDS at the particle surface of the 1% APO sample. This P-rich CEI layer is formed for two reasons: i.) a P-containing layer already formed at the particle surface in the pristine 1% APO sample as discussed above in Fig. S5; ii.) the interaction between the cathode and the electrolyte during cycling can further form P-containing CEI layer. Compared to the P signal distribution on the primary particles of bare NCM9244, the observed P-rich layer in this 1% APO sample is mainly contributed from the Li3PO4 coating layer, which may lead to its enhanced structural stability.
Figure 6. STEM characterization on (a)–(e) the bare NCM9244 after 200 cycles and (f)–(j) the 1% APO cathode after 200 cycles. (a) Cross-sectional STEM-HAADF image (the scale bar corresponds to 5 nm) at a primary particle edge in the cycled bare NCM9244 sample along with the corresponding (b) STEM-EDS Ni/Co/Mn mapping and (c) STEM-EDS P mapping, the STEM-EDS spectrum in (d) is from the bottom part of (a) inside the particle and the STEM-EDS spectrum in (e) is from the particle edge. (f) Cross-sectional STEM-HAADF image (the same scale as (a)) at a primary particle edge in the cycled 1% APO cathode sample along with the corresponding (g) STEM-EDS Ni/Co/Mn mapping and (h) STEM-EDS P mapping, the STEM-EDS spectrum in (i) is from the bottom part of (f) inside the particle and the STEM-EDS spectrum in (j) is from the particle edge.
Download figure:
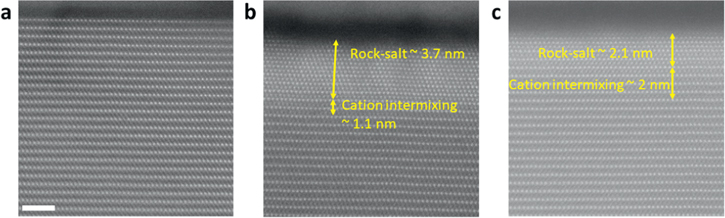
Standard image High-resolution imageFigure 7. Cross-sectional STEM-HAADF images at the particle surface to show the reconstruction layer in (a) pristine 1% APO cathode, (b) bare NCM9244 after 200 cycles, and (c) 1% APO cathode after 200 cycles. The scale bar in (a) corresponds to 2 nm and applies to (b) and (c).
Download figure:
Standard image High-resolution imageSince the reconstruction layer at the particle surface plays an important in Li transport during cycling, the particle surface is studied by directly visualizing the atomic structure for the samples of pristine 1% APO, 200-cycled 1% APO and 200-cycled bare NCM9244. Figure 7a shows that the pristine state of 1% APO cathode exhibits a well-ordered layered structure along the [010] zone axis without any surface reconstruction. High-nickel cathode materials are expected to experience surface reconstruction upon cycling. During the delithiation process (charge to 4.3 V), the Li-ion extraction leads to the formation of the NiO2-like rock-salt phase. As a result, the stacking sequence of oxygen layers changes from cubic close-packing (ABCABC) to hexagonal close-packing (ABAB). This stacking sequence change induces the irreversible rock-salt phase formation and further causes the degradation of high-nickel cathode material. 31,32 Because of the importance of the surface reconstruction, the atomic structures at the particle surface are compared between 200-cycled bare NCM9244 (Fig. 7b) and 200-cycled 1% APO cathode (Fig. 7c). A reconstruction layer composed of rock-salt phase and cation intermixing is observed in both cycled samples, but in a different fashion. The total thickness of the reconstruction layer in the cycled bare NCM9244 is 4.8 nm composed of a 3.7 nm thick rock-salt phase and 1.1 nm thick cation intermixing region. In contrast, the reconstruction layer in the cycled 1% APO cathode sample is comparatively alleviated in terms of (i.) the reconstruction layer total thickness reduces to 4.1 nm, ∼15% thinner than the total thickness of the bare NCM9244 sample reconstruction layer; (ii.) the rock-salt layer is 2.1 nm thick which is only 57% of the rock-salt layer thickness in the bare NCM9244 sample. This comparison suggests that the Al doping along with Li3PO4 coating using the AlPO4 coating method helps stabilize the layered structure of NCM9244 and partially prevents stacking sequence change of oxygen layers, which protects and prolongs the cycle life of high-nickel cathode materials.
Conclusions
AlPO4 nanoparticles are successfully synthesized and used to coat high-nickel NCM9244 cathode precursors via a dry coating approach. Upon heat treatment forming layer-structured NCM9244 cathode materials, the AlPO4 coating layer is transformed to Li3PO4 coating while the Al element is doped in the bulk structure of the high-nickel cathode material, playing dual protective functions of surface coating and cation doping. The new AlPO4 coating method effectively prevents particle crackings and enhances stable surface structure by Al doping and inhibits undesirable reactions with the electrolyte and its decomposition product by the phosphate surface coating, leading to significantly improved cycling stability. The coating method provides a facile and environmentally friendly approach toward improving the performance and accelerating the scale-up of high-nickel cathode materials for Li-ion batteries.
Acknowledgments
This work is supported by the Department of Energy, Office of Energy Efficiency and Renewable Energy (EERE), Vehicle Technology Office, under Award no. DE-EE0008447. The TEM work was conducted in the William R. Wiley Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by DOE's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory (PNNL). PNNL is operated by Battelle for the Department of Energy under Contract DE-AC05–76RL01830.