Abstract
Improved performance of lithium-ion batteries (LIBs) plays a critical role in the future of next- generation battery applications. Nickel-rich layered oxides such as LiNi0.8Mn0.1Co0.1O2 (NMC 811), are popular cathodes due to their high energy densities. However, they suffer from high surface reactivity, which results in the formation of Li2CO3 passive layer. Herein, we show the role of nanosecond pulsed laser annealing (PLA) in improving the current capacity and cycling stability of LIBs by reducing the carbonate layer, in addition to forming a protective LiF layer and manipulating the NMC 811 microstructures. We use high-power nanosecond laser pulses in a controlled way to create nanostructured surface topography which has a positive impact on the capacity retention and current capacity by providing an increased active surface area, which influences the diffusion kinetics of lithium-ions in the electrode materials during the battery cycling process. Advanced characterizations show that the PLA treatment results in the thinning of the passive Li2CO3 layer, which is formed on as-received NMC811 samples, along with the decomposition of excess polyvinylidene fluoride (PVDF) binder. The high-power laser interacts with the decomposed binder and surface Li+ to form LiF phase, which acts as a protective layer to prevent surface reactive sites from initiating parasitic reactions. As a result, the laser treated cathodes show relative increase of the current capacity of up to 50%, which is consistent with electrochemical measurements of LiB cells.
Export citation and abstract BibTeX RIS
Lithium-ion batteries (LIBs) have revolutionized portable electronics in the last three decades, as they are able to deliver higher energy per unit volume or mass than other rechargeable battery systems. 1 The higher energy is derived from high cell voltage and specific capacity, both of which depend upon materials chemistry and microstructural characteristics of cathode and anode materials. The oxide cathode materials, which have shown the highest performance in terms of energy density so far, can be classified into the following three categories: (1) 2-D layered LiCoO2 related materials; (2) 3-D spinel LiMn2O4 related materials; and (3) 1-D olivine LiFePO4 related materials. 2
The layered LiCoO2 cathode, originally commercialized by Sony Corp., has played a central role in portable electronics including cell phones and laptop computers. However, the high cost associated with cobalt, availability, toxicity, chemical instability, and safety concerns have prevented its usage in transportation and stationary storage applications. 3 In LiCoO2, Li+ and Co3+ are ordered in the alternate (111) planes of the NaCl structure with a cubic close-packed array of oxide ions. This structure is referred to as the O3 structure, where large differences in charge and size between Li+ and Co3+ cations lead to two-dimensional cation ordering, which is critical to fast two-dimensional lithium-ion diffusivity and ionic conductivity. The lithium-ion diffusion in the (111) plane occurs from one octahedra site to another via neighboring tetrahedra which share faces with three octahedra, providing low diffusion migration energies. 4 The chemical instability results from the overlap of the Co3+/4+:3d band with the top of the O2−:2p band, which leads to oxygen release on Li+ ion charging beyond 50%. This also limits the current capacity in LiCoO2 to about 140 mAhg−1. On the other hand, Ni and Mn doping lower chemical instability and safety concerns, because the Ni3+/4+:3d band barely touches the top of the O2−:2p band, and Mn3+/4+:3d band lies well above the top of the O2−:2p band. These considerations have led to the development of LiNixMnyCo(1-x-y)O2 cathodes, which offer better safety, in addition to lower cost of Ni and Mn than Co. 5
Here, we focus on LiNixMnyCozO2 cathode with x = 0.8, and y = z = 0.1, which is known as NMC 811. The NMC 811 can reach a theoretical specific current capacity of 200 mAhg−1 with upper cut off voltage of 4.3 V vs Li/Li+. However, it suffers from the following limitations: (1) They are reported to show structural changes from the layered phase (R m) to the spinel-like phase (Fd
m) to the spinel-like phase (Fd m) and rock salt phase (Fm
m) and rock salt phase (Fm m) during high voltage cycling and at high temperatures.
6–9
This structural degradation and electrolyte oxidation at the cathode surface results in poor cyclability.
10
(2) During cycling, the migration of transition metal ions (Ni2+) to the lithium ions sites results in oxygen and lithium ions loss from the surface, which damages the structure, resulting in the capacity loss.
11
(3) There is a significant Li/Ni cation mixing because of similar radii of Li+ (0.076 nm) and Ni2+ (0.069 nm), which slows lithium diffusion.
12
(4) In addition, the cathode surface is easily contaminated by the LiOH and Li2CO3 layer formation due to its high surface reactivity, which reduce its performance.
13–15
So far various techniques like coatings, doping, electrolyte additives, have been used to overcome these issues to improve the cathode performance.
16–22
Previous studies on laser processing involved surface restructuring and patterning to enhance electrolyte interaction with modest increase in current capacity.
23–26
In this work, we focus on nanosecond pulsed laser annealing (PLA), which was pioneered by Narayan et al.
27,28
for defect engineering and surface modification. Recently, we have used PLA to improve current capacity and long-term cyclability in LMNO cathodes and graphite anodes.
29,30
m) during high voltage cycling and at high temperatures.
6–9
This structural degradation and electrolyte oxidation at the cathode surface results in poor cyclability.
10
(2) During cycling, the migration of transition metal ions (Ni2+) to the lithium ions sites results in oxygen and lithium ions loss from the surface, which damages the structure, resulting in the capacity loss.
11
(3) There is a significant Li/Ni cation mixing because of similar radii of Li+ (0.076 nm) and Ni2+ (0.069 nm), which slows lithium diffusion.
12
(4) In addition, the cathode surface is easily contaminated by the LiOH and Li2CO3 layer formation due to its high surface reactivity, which reduce its performance.
13–15
So far various techniques like coatings, doping, electrolyte additives, have been used to overcome these issues to improve the cathode performance.
16–22
Previous studies on laser processing involved surface restructuring and patterning to enhance electrolyte interaction with modest increase in current capacity.
23–26
In this work, we focus on nanosecond pulsed laser annealing (PLA), which was pioneered by Narayan et al.
27,28
for defect engineering and surface modification. Recently, we have used PLA to improve current capacity and long-term cyclability in LMNO cathodes and graphite anodes.
29,30
This paper addresses microstructural and defect engineering of NMC 811 cathodes by using nanosecond pulsed laser annealing (PLA) to improve their performance and current capacity. Herein, we use PLA treatment for thinning and removal of the carbonate layer, which is generally formed on the cathodes surface due to the high reactivity of surface Li2O with exposure to oxygen and CO2, and removal of excess polyvinylidene fluoride (PVDF) binder. During the laser treatment, the surface Li reacts with the decomposed binder to form LiF, which acts as a protective layer to prevent the unwanted side reactions with the electrolyte. 6 Such a surface LiF phase helps in reduction of the electrochemical oxidative reactions of carbonate solvents, and generation of transition-metal ions and acidic crossover species, which in turn improve the performance of cells. 30 We also created nanostructured surface topography on the laser treated samples, which has a positive impact on the capacity retention and current capacity by providing an increased active surface area. An increased active surface area by nanostructuring influences the diffusion kinetics of lithium-ions in the electrode materials through the electrolyte. Furthermore, PLA treatment also creates both cation and anion vacancies, where cation vacancies promote lithium-ion mobility, which outweigh harmful effects related to anion vacancies promoting cation mixing. For future battery manufacturing, a balanced combination of defect engineering and microstructure optimization, will enable the development of a new generation of high power and high energy lithium-ion cells.
Experimental
The NMC 811 samples were annealed using nanosecond ArF excimer laser (wavelength = 193 nm, pulse duration = 20 ns) at 0.2–0.4 J cm−12 laser energy density with variable (5–10) shots. However, the optimized pulse energy density was found to be 0.3 J cm−12, therefore, we present results for 0.3 J cm−12. There was a significant change in the morphologies observed in laser annealing samples compared to the reference samples. The samples were characterized by Scanning electron micrograph (SEM), X-ray Diffraction (XRD), for structure and surface morphology. HR-SEM for surface morphology was performed using FEI Verios 460 L SEM. The XRD 2θ scans were performed using a Rigaku SmartLab X-ray diffractometer in Brag diffraction operating mode, using a Cu-Ka radiation source from a sealed tube operating at a voltage and current of 40 kV and 25 mA, respectively, and state-of-the-art LENXEYE XE detector. The X-ray photoelectron spectroscopy (XPS) was performed for atomic bonding analysis. Surface chemical analysis was carried out using X-ray photoelectron spectroscopy (XPS) was done on a SPECS XPS system (SPECS FlexMod XPS) using a high-resolution PHOIBIS 150 hemispherical analyzer. Measurements were taken at a base pressure of 2 × 10−10 mbar with Mg kα excitation source (1254 eV). The takeoff angle was at normal to the sample surface. Energy calibration was done by referencing to adventitious carbon C 1 s line at 285 eV binding energy.
NMC811 powder, polyvinylidene fluoride (PVDF) binder, and C45 conducting carbon were purchased from MTI Corporation. N-methyl-pyrrolidone (NMP) solvent was purchased from Aldrich and used as received. NMC811 electrode was prepared by mixing the NMC811 powder with C45 and PVDF in NMP in a weight ratio of 90:5:5 to form a well homogenized slurry, which was then cast onto aluminum foil with a doctor blade and left under a UV light to allow the solvent (NMP) to evaporate. The electrode was then dried in a vacuum oven overnight at 110 °C before cutting into circular discs with an area of 1.27 cm2 and transferred to an Ar filled glove box. The electrodes have an active material loading of approximately 3.0–4.0 mg cm−2. More details of the electrode preparation has been published previously. 6 Coin cells were assembled with NMC811 as the cathode, Li as the anode, Celgard 2320 as the separator and 1.2 M LiPF6 dissolved in a mixture of ethylene carbonate (EC) and ethyl methyl carbonate (EMC) at a weight ratio of 3:7 as the electrolyte inside an Ar filled glovebox with the oxygen and water levels below 0.5 ppm. Cyclic voltammograms (CVs) were recorded on a Biologic VSP instrument in the voltage range of 3.0–4.5 V at a scan rate of 0.1 mV s−1. Electrochemical impedance spectra (EIS) were tested on the same instrument with AC signal amplitude of 10 mV in the frequency range from 200 kHz to 10 mHz. Galvanostatic charge/discharge was carried out on an Arbin instrument at C/10 (1 C = 200 mAh g−1) for three cycles, followed by cycling at C/2 for 300 cycles at room temperature.
Results and Discussion
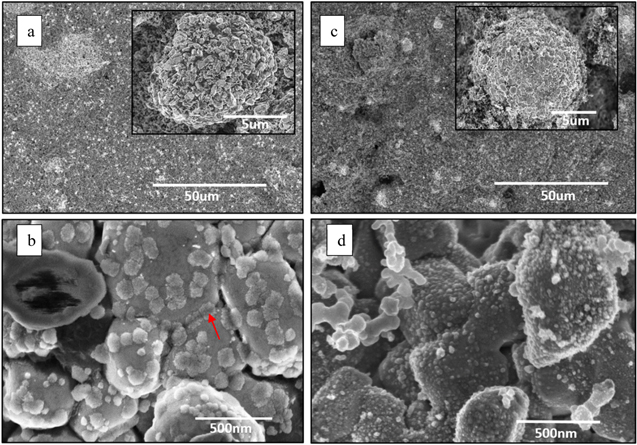
We carried out high-resolution SEM studies to study the microstructural changes and surface morphology. Figure 1a shows that as-received NMC 811 samples consist of LiNi0.8Co0.1Mn0.1O2 phase having primary average grain size of about 500 nm over a large area. These NMC 811 grains are mixed with carbon additive and PVDF binder to enhance electrical conductivity and facilitate cell manufacturing. At a higher magnification in as-received samples as shown in Fig. 1b, we see a primary NMC 811 phase and a secondary phase of residual lithium grains, in addition to lithium carbonate (Li2CO3) thin layer. These lithium grains have an average grain size about 100 nm on the top of the primary 500 nm grains samples. It is envisaged that Li2CO3 thin layer forms from the residual surface Li2O reaction with CO2 during processing (Li2O(s) + CO2 (g) → Li2CO3 (s)). The formation of this carbonate layer (denoted by arrows in the Fig. 1d is attributed to the degradation of cathode performance. A severe accumulation of surface carbonates, which are poor conductors, is responsible for electrolyte consumption, Li depletion and thicker SEI (Solid Electrolyte Interphase) which results in deterioration of cell capacity. 31
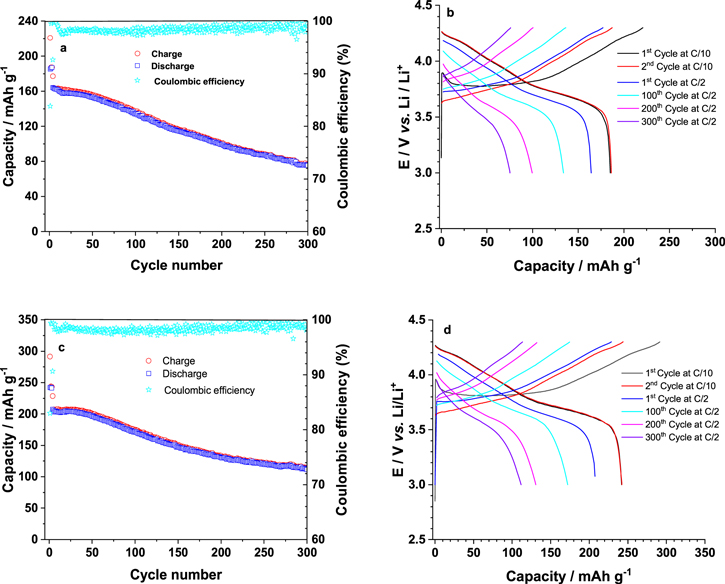
Figure 1. SEM images of the (a) as-received NMC 811 at a low magnification; (b) formation of Li and Li2CO3 at higher magnification; (c) formation of pits after laser annealing; and (d) removal of Li and Li2CO3 and formation of nanostructured grains on the top of original micrograins in laser annealed NMC 811 sample.
Download figure:
Standard image High-resolution imageAfter the laser treatment (energy density = 0.3Jcm−2), we see pits (dark regions), where PVDF binder is evaporated off, as shown in Fig. 1c. It is surmised that the creation of pits is useful in enhancing the electrolyte interactions as more Li-ion pathways are available from the electrolyte phase to active material due to the presence of nanopores, thereby, increasing the performance of cells. At a higher magnification (Fig. 1d), we see that the lithium grains and the lithium carbonate layer have been mostly evaporated in the laser treated samples. On exposing the samples with high-energy laser (ArF, bandgap = 6.4 eV), the Li2CO3 (3.55 eV indirect and 5.10 eV direct bandgap) 32 layer decomposes resulting in the thinning of the carbonate layer. Furthermore, the PLA treatment initiates the reaction between the surface residual Li+ species and decomposed PVDF binder, causing the formation of a surface LiF phase. 30,33 This LiF acts as a passive protective layer. Interestingly, we find nanostructuring of LiNi0.8Co0.1Mn0.1O2 grains into finer grains with an average size of 15 nm. It is suggested that the excimer laser melted the top surface of the grains and with ultrafast quenching at the room temperature, it was recrystallized. The nanostructuring of NMC 811 particles increases the surface area and provides more available surface reaction area and consequently the availability of more active sites for the electrolyte interaction.
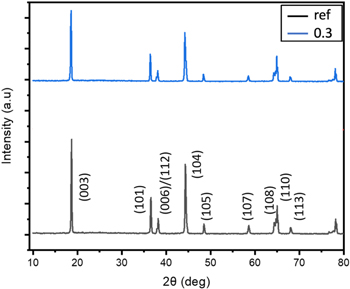
The structural ordering of the samples was quantitatively analyzed using the XRD measurements. The XRD spectra indicate that both the reference NMC 811 and laser annealed NMC 811 are single-phase layered rhombohedral Li(NiCoMn)O2 with R-3M space group (JCPDS reference number: 01–080–7011). 34 No other peaks are detected in the annealed sample, indicating that PLA treatment did not alter the bulk NMC811 structure. The structure of nanostructured NMC811 also has the same structure, but stoichiometry and defect content are different from the (reference) as-received sample. We did not detect the presence of Li and Lithium Carbonate layer in the XRD because of low scattering efficiency from Li and the carbonate layer may be too thin to be detected. Furthermore, in Ni-rich cathodes, cation mixing between Li and Ni may occur, resulting in the heavier Ni cations occupying Li sites, which causes a weaker intensity of (003) peak as seen in Fig. 2. 35,36 This cationic disordering in the layered structures of Ni-rich oxides is attributed to the rapid quenching due to the melt, oxygen vacancies, and migration of reduced Ni2+ ion into the lithium layers because of the similar ionic radii of Ni2+ (0.69 Å) and Li+ (0.76 Å). 37 The ratio of the integrated intensity for the (003) and (104) reflections, I003/I104, signifying cation mixing 38 and Ni/Li disordering is 1.35, and 1.31 for the reference and 0.3 J cm−2, respectively. It is envisaged that the laser annealing results in the formation of anion and cation vacancies. These cation vacancies provide extra pathways for Li+ ions for charging and discharging. It should be mentioned that anion vacancies contribute to disorder. 39–41 However, this disorder is not that significant. There is an optimum balance between the defect concentration and cation mixing. 42 In addition, clear splitting of the (108)/(110) diffraction peaks for all the samples show that the highly ordered layered structure was not influenced with this small amount of substitution.
Figure 2. XRD spectra of the reference sample and multiple shots laser annealed sample at 0.3 J cm−2.
Download figure:
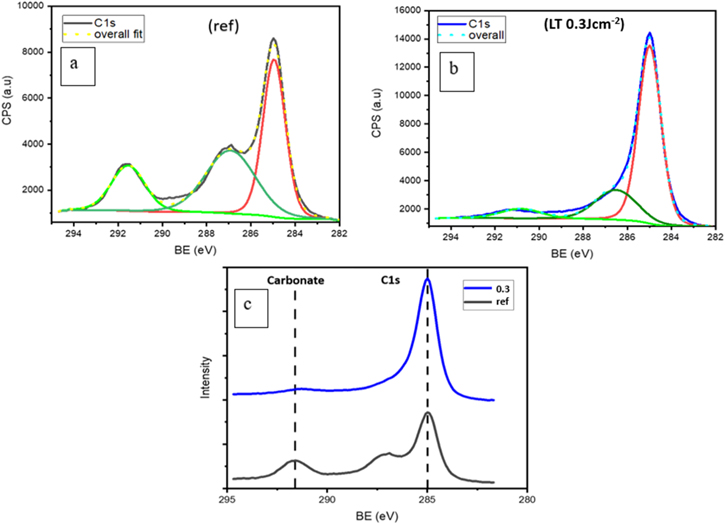
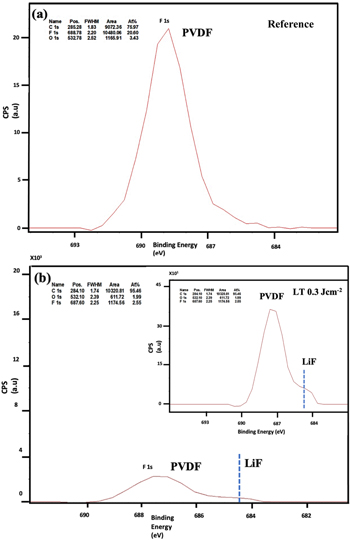
Standard image High-resolution imageThe reference and laser annealed NMC 811 samples were further characterized by X-ray photoelectron spectroscopy (XPS) to understand surface chemistry evolution. Figures 3a,3b shows the C 1 s spectra of the reference and 0.3 J cm−2 laser annealed sample. There is an adventitious carbon peak at 284.9 eV 43 along with a dominant carbonate peak at 290.8 eV and PVDF peak 286.9 eV observed in the reference sample as shown in Fig. 3a. It is reported that NMC 811 has very high surface reactivity. Due to the reduction of Ni3+ to Ni2+, active oxygen species are generated at the cathode surface, which react in the presence of air and moisture to form carbonate and hydroxide impurities, 44 as observed in SEM images as well. As mentioned, these Li2CO3 impurities are responsible for degradation phenomena and capacity fade in NMC 811 cell. The XPS results, further confirm the thinning of carbonate and the PVDF binder in the laser annealed samples as shown in Fig. 3c. This decrease of fluorine from the surface, which acts as a blockage for Li+ ions to interact during the charging, is also seen in F 1 s spectra in Fig. 4b, suggesting that PLA results in decomposition of the PVDF binder. Furthermore, the presence of LiF peak at 685 eV in Fig. 4b reveals the formation of this new surface phase by the reaction between the residual surface Li and PVDF binder during laser annealing. This peak is absent in the reference sample (Fig. 4a).
Figure 3. C1s fitted XPS spectra of the (a) as-received ref sample and (b) 5 shots laser annealed sample at 0.3 J cm−2, (c) Comparison of C1s spectra showing the decrease in carbonate peak post laser annealing NMC 811sample.
Download figure:
Standard image High-resolution imageFigure 4. F1s fitted XPS spectra of the (a) reference sample showing significant PVDF peak and (b) laser annealed sample at 0.3J/cm2 showing decrease in PVDF peak and origination of LiF peak at 685eV. The inset shows higher magnification of LiF peak.
Download figure:
Standard image High-resolution imageThe electrochemical performance of the baseline and laser treated NMC811 samples were evaluated by first cycling under a low current rate of C/10 for three cycles, followed by cycling at a high current rate of C/2 between the voltage range of 3.0 and 4.3 V. Figures 5a and 5c show the long cycling performance of the pristine and laser treated NMC 811 at 0.3 J cm−2 while Fig. 5b and 5d compare the charge (delithiation)/discharge (lithiation) profile of the 1st, 2nd cycle at C/10, and the 1st, 100th, 200th and 300th cycle at C/2 for the pristine and laser treated NMC 811 at 0.3 J cm−2. The initial charge/discharge capacities of the baseline NMC811 at C/10 (1 C = 200 mAhg−1) are 220.8/185.1 mAh g−1, which is enhanced to 291.5/242.0 after laser annealing NMC 811 at 0.3 J cm−2. The laser annealing treatment is seen to increase the capacity of the treated NMC811 samples significantly. It is envisaged that the near surface nanostructuring and creation of cation vacancies play a critical role in enhancing the capacity of the cell. Recent results proved that during discharging the lithium concentration at cathode side is significantly increased on the top and along the contour of the free-standing microstructures, providing additional Li pathways and capacities. 45,46 For cycling at high current rate of C/2, the initial charge/discharge capacity in the reference NMC 811 sample is only 177.1/164.1 mAhg−1, which drops to 76.0/75.3 mAhg−1 after 300 cycles. Remarkably, in the laser-treated NMC 811 samples, the initial charge/discharge capacity improves to 228.7/207.3 mAhg−1 and it decreases to 113.6/111.7 mAhg−1 after 300 cycles. This improvement after 300 cycles is over 48% suggesting significant enhancement in the NMC 811 performance. These results indicate that the laser treatment exposes more reactive sites and shortens the Li+ diffusion length and thus, enhances the capacities of the treated samples. The sample treated at 0.3 J cm−2 with 10 shots offers the best combination of higher reversible capacity and better capacity retention. Thus, the laser treatment effectively increases the current capacity of the LIB compared to the non-treated samples.
Figure 5. Charge/discharge capacities and profile of NMC 811 (a), (b) baseline and laser annealed at (c), (d) 0.3 J cm−2.
Download figure:
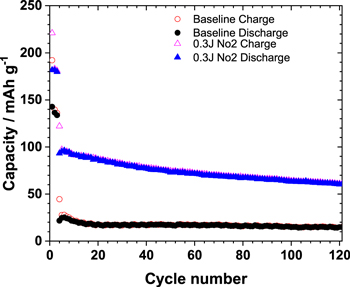
Standard image High-resolution imageIt should be mentioned that the moisture and prolonged storage of these electrodes results in a severe accumulation of surface carbonates. 47,48 The carbonate species produced during ambient exposure of NMC 811 are electrochemically inactive with poor electronic conductivity, which hinders Li diffusion significantly, 49,50 resulting in the capacity fade as seen in Fig. 6. Figure 6 shows the long cycling performance of 8 months old NMC 811 sample vs its performance after the laser treatment. As suggested by the reports, it is seen that the cell performance degrades. The initial charge/discharge capacities of the baseline NMC811 at C/10 (1 C = 200 mAhg−1) are 192 and 142 mAh g−1. However, post laser treatment at C/10, the charge/discharge capacities are 221 and 181 mAh g−1, respectively. This is in agreement with the proposed effect of laser annealing the NMC 811 samples in improving the performance of LIB cathodes for longer cycles due to the removal of carbonate layer, which leads to increase in resistance, impeding the charge transfer and the mobility of lithium ions and electrons for the electrochemical redox reaction. The laser treatment in terms of pulses can be varied based on the storage conditions and thickness of the carbonate layer in the samples.
Figure 6. Charge/discharge capacities of the 8 months aged (a) baseline (reference NMC811) and laser treated samples at 0.3 J cm−2.
Download figure:
Standard image High-resolution imageConclusions
We have successfully demonstrated the role of Pulsed Laser Annealing in improving the performance of NMC 811 cathodes. On treating the cathode samples with ultrafast laser pulses of optimum energy density, the traditional carbonate layer, which is rapidly formed due to high surface reactivity of the cathode in presence of moisture and CO2, is significantly reduced. As a result, we see higher capacity rates and capacity retention in the laser treated cells. Additionally, this treatment initiates reaction between the partial decomposition of PVDF binder, which interacts with the lithium to form LiF layer. This layer reduces generation of dissolved transition metal ions and acidic cross-over species, which prevent the structural degradation of the cell. Also, the laser treated cells have nanostructured topography within individual grains which provides enhanced surface area for electrolyte interaction, thereby, increasing the Li+ diffusion kinetics of the cell. The initial charge/discharge capacity in the reference NMC 811 sample is only 177.1/164.1 mAhg−1, which drops to 76.0/75.3 mAhg−1 after 300 cycles. Remarkably, in the laser-treated NMC 811 samples, the initial charge/discharge capacity improves to 228.7/207.3 mAhg−1 and it decreases to 113.6/111.7 mAhg−1 after 300 cycles. This improvement after 300 cycles is over 48% suggesting significant enhancement in the NMC 811 performance. Since, the NMC 811 electrodes have high surface reactivity, therefore it is important to vary the number of laser pulses and density accordingly. For future battery manufacturing, a balanced combination of defect engineering and microstructure optimization, will enable the development of a new generation of high power and high energy lithium-ion battery.
Acknowledgments
This work was supported by the National Science Foundation Grant (DMR-2016256). The comments and discussions with Professor John Prater are gratefully acknowledged. Battery testing research (XS and MPP) was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Materials Sciences and Engineering Division under contract number DE-AC05–00OR22725. This work was performed in part at the Analytical Instrumentation Facility (AIF) at North Carolina State University, which is supported by the State of North Carolina and the National Science Foundation (award number ECCS-2025064). The AIF is a member of the North Carolina Research Triangle Nanotechnology Network (RTNN), a site in the National Nanotechnology Coordinated Infrastructure (NNCI).
This manuscript has been authored by UT-Battelle, LLC, under contract DE-AC05–00OR22725 with the US Department of Energy (DOE). The US government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).