Abstract
In recent years, electrochemical biosensors based on semiconductor and metal nanostructures have attracted a great deal of attention as new instruments in the healthcare arsenal that could substantially enhance early diagnostics capabilities and thus enable active health management. Among numerous materials studied, nanostructured ZnO has been recognized as a promising platform for biomedical applications owing to its low cost, relative ease of preparation leading to a rich variety of nanostructures with high aspect ratios (nanowires, nanobelts, nanoflakes), proven biocompatibility in the bulk form, electronic properties supporting various device types, and catalytic surface activity. In this contribution, we review the recent progress in development of enzymatic and non-enzymatic biosensors based on ZnO nanostructures. After a critical discussion of biocompatibility of nanostructured ZnO, we segue into the discussion of ZnO-based devices for detection of physiologically important analytes, including glucose, cholesterol, L-lactic acid, uric acid, metal ions, and pH. Special attention is given to ZnO nanorod based sensors for intracellular measurements.
Export citation and abstract BibTeX RIS
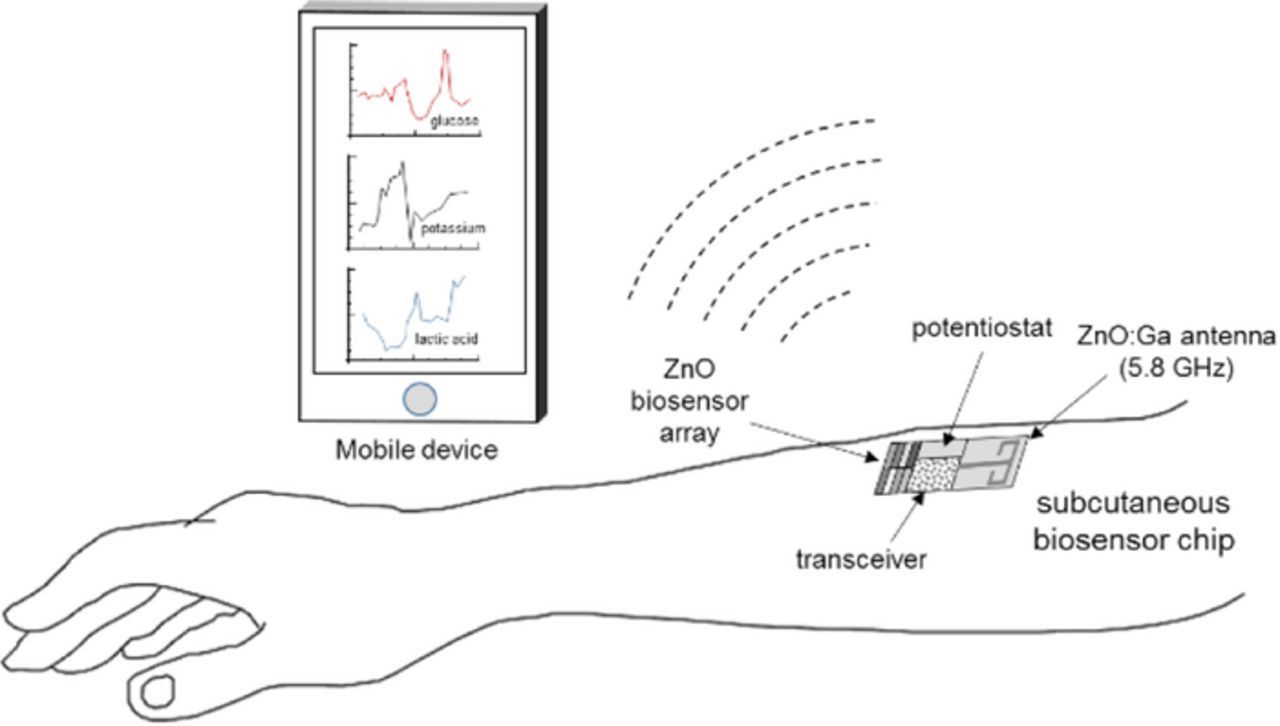
Ongoing transformation of healthcare from reactive and hospital-centered to preventive and person-centered mandates development of ever new technologies that would enable long-term continuous monitoring of all pertinent physiological parameters. As a result, early diagnostics capabilities would be enhanced substantially, and in the process pave the way for active management of one's health in part by causing appropriate adjustment to one's lifestyle. Among the critical physiological parameters requiring continuous monitoring are blood levels of glucose, cholesterol, uric acid, lactic acid, as well as calcium, iron, magnesium, and potassium ions. The most effective way to monitor these parameters is through subcutaneous sensors, which can provide high sensitivity and accuracy owing to their direct access to the interstitial fluid, unlike noninvasive technologies such as bioimpedance, infrared, Raman, optical coherence, etc. However, current state-of-the-art implantable sensors, e.g. for glucose monitoring, have limited functional longevity (of the order of weeks) due to degradation and fouling from fibrosis and inflammation. It is thus imperative to develop biocompatible biosensors that can remain functional in the body for an extended period (>1 year). Such long-term and miniature biosensors combined with control electronics and wireless telemetry (laboratory on an implantable chip (LOIC)-see Fig. 1) would help realize preventive and person-centric healthcare, replacing the need for frequent lab-based blood tests and superfluous dependence on medications.
Figure 1. A conceptual description of the subcutaneous "lab on an implantable chip" with wireless telemetry.
As alluded to briefly above, electrochemical biosensors are more efficient than devices based on other measurement techniques such as nuclear magnetic resonance (NMR) spectroscopy,1 radioisotope tracing,2,3 and microfluorometric assays.4 The advantages of electrochemical sensors include high sensitivity, wide range of detection, real-time monitoring capability, ease of fabrication and control, reproducibility, and low cost. Among the most common electrochemical techniques employed in biosensors5 (potentiometry, cyclic voltammetry, differential pulse voltammetry, electrochemical impedance spectroscopy), the amperometry has become the most popular method as it provides a linear relationship between the sensor output and analyte concentration.6 Further details on the operation modes of the electrochemical sensors can be found elsewhere.7 Potentiometric sensors are also frequently used; however, they typically have lower sensitivity compared to the amperometric varieties because of the semi-logarithmic relationship between the measured signal and analyte concentration.6
ZnO has a long history of sensor applications in the form of bulk crystals, thin and thick films, and sintered pellets.8–11 Recently, ZnO-based nanostructures (nanowires, nanorods, tetrapods, nanotubes, nanospheres etc.) have attracted particular attention owing to their high sensitivity to chemical species warranted by very large surface-to-volume ratio. They have been explored for sensing humidity,12 pH,13 and a variety of gases, including C2H5OH,14 H2S,15 NO,16 NO2,17 NH3,18 etc. High sensitivity of nanostructured ZnO to important constituents in blood as well as the interstitial fluid, including glucose,19–21 cholesterol,22,23 uric acid,24,25 L-lactic acid,26,27 potassium,28 sodium,29 calcium,30,31 and magnesium32 ions, makes this material of particular interest for biomedical applications. The motivation behind the use of nanostructures is the increase surface-to-volume ratio (keeping the sensor size small) which enhances their reactivity, which in turn allows them to translocate more easily through cell membranes (if not bound to a substrate), efficiently bind molecular species, and catalyze chemical reactions.33 ZnO nanostructures can be formed in various shapes, such as nanowires, nanobelts, nanotubes, and nanorings faceted with polar {0001} or nonpolar { } (a-plane) and {
} (a-plane) and { } (m-plane) faces.34 The nanostructures are produced by a variety of techniques, among them are vapor-phase transport via catalyst-assisted vapor–liquid–solid (VLS)35,36 or catalyst-free vapor–solid processes,37,38 hydrothermal based chemical approach,38,39 gas reactions,40 catalysis-driven selective molecular beam epitaxy (MBE),41 metal organic chemical vapor deposition (MOCVD),42 solution growth,43 and electrochemical deposition.44
} (m-plane) faces.34 The nanostructures are produced by a variety of techniques, among them are vapor-phase transport via catalyst-assisted vapor–liquid–solid (VLS)35,36 or catalyst-free vapor–solid processes,37,38 hydrothermal based chemical approach,38,39 gas reactions,40 catalysis-driven selective molecular beam epitaxy (MBE),41 metal organic chemical vapor deposition (MOCVD),42 solution growth,43 and electrochemical deposition.44
Biocompatibility of ZnO Nanostructures
ZnO in its bulk form is considered as a biocompatible and nontoxic material; however, only limited data exist on the biodegradability and biocompatibility of ZnO nanostructures, and, as a consequence, their interaction with biological systems still remains unclear.45 Even though ZnO nanoparticles have been approved by the FDA for cosmetic uses, the detailed toxicological profile and the mechanisms of cytotoxicity are not yet well understood.
Although Zn2+ is an indispensable trace element for humans with recommended daily dietary allowance ranging from 2 mg in infants to 11 mg for males of age 14 and higher (the tolerable intake values range from 4 mg in infants to 40 mg for ages 19 and higher) (see Table I),46 toxicity concerns are related to possible elevated concentrations of Zn2+ due to dissolution of ZnO nanostructures under acidic or strongly basic conditions. Although most of the studies report that the cytotoxicity of ZnO nanoparticles, which mainly originates from induction of oxidative stress, is due to their dissolution in the extracellular region (see review by Rob and Wim47 and references therein), some suggest that ZnO nanoparticles are taken up by the cells and their dissolution occurs in the intracellular region.48 Absorption of ZnO nanoparticles has been reported to result in elevated levels of zinc in liver, adipose tissue, and pancreas.49
Table I. Recommended and tolerable daily zinc intake values.
| Recommended dietary allowance | Male: 2 mg (0–6 months), 3 mg (7 months–3 years), 5 mg (4–8 years), 8 mg (9–13 years), 11 mg (14+ years) | Female: 2 mg (0–6 months), 3 mg (7 months–3 years), 5 mg (4–8 years), 8 mg (9–13 years), 9 mg (14–18 years, 12 mg during pregnancy, 13 mg during lactation), 8 mg (19+ years, 11 mg during pregnancy, 12 mg during lactation) |
| Tolerable upper intake values | Male or female: 4 mg (0–6 months), 5 mg (7–12 months), 7 mg (1–3 years), 12 mg (4–8 years), 23 mg (9–13 years), 34 mg (14–18 years), 40 mg (19+years) | |
It was also found that ZnO nanorods are capable of reducing adhesion and viability of various cell types.50–52 Comparing adhesion and viability of macrophages on ZnO nanorods, flat ZnO films, and glass substrates, Zaveri et al.50 have found that that cell adhesion and viability correlate to both nanotopography and elevated concentrations of Zn2+ dissolved in the cell culture media. The reduced cell viability on both flat and nanostructured ZnO substrates was related to ZnO dissolution, and the amount of dissolved Zn was found to be significantly higher for ZnO nanorods compared to flat ZnO substrates. The number of adherent macrophages was the lowest on ZnO nanorods and the highest on glass, and the number of live cells on the surfaces was revealed to decrease with an increase in Zn concentration. It should be mentioned that the nanotopography factor cannot be ruled out, because Lee et al.53 observed reduced adhesion and survival of endothelial cells and fibroblasts on ZnO nanorods coated with SiO2 to prevent the leaching of ZnO into a solution. In this case, the reduced adhesion and viability was attributed to a lack of sustainable adhesive complexes on nanorod substrates. Jansson et al.52 studied adhesion and viability of bacteria (Pseudomonas aeruginosa and Staphylococcus epidermidis) on nanostructured ZnO in comparison to flat ZnO films and glass substrates. Both ZnO nanorod and sputtered film ZnO surfaces demonstrated the bactericidal effect on adherent P. aeruginosa, the numbers of dead bacteria on nanostructured and flat ZnO surfaces were 2.5 and 1.7 times larger than that on glass, respectively. The bactericidal effect of ZnO on S. epidermidis was even more significant, with flat ZnO and ZnO nanorod surfaces killing ∼20 and 30 times more bacteria than the glass surface, respectively. Thus, the nanostructured ZnO shows the highest bactericidal ability. The difference in bactericidal activity against the two types of bacteria was attributed to structural differences in the cell walls of these strains, which may results in different interaction with the ZnO surface. The dissolution of ZnO with the release of Zn2+ ions and physical penetration of the nanorods into bacterial cells54 was considered as reasons of high antibacterial activity of the nanostructured ZnO surface.
Exploration of ZnO dissolution in biofluids has great significance for its applications in biomedical devices, because the degradation rate for ZnO is closely related to its biocompatibility and cytotoxicity. Moreover, the sensor material should feature a reasonable time of functionality in biological systems. While a device "survival lifetime" of a few hours would suffice for diagnosing acute diseases, a lifetime of several months is necessary for continuous monitoring.
In aqueous media, the expected dissolution chemistry55 for ZnO nanostructures is
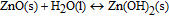

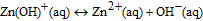
making the net reaction
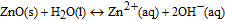
As evidenced from the reaction, Zn ions are produced in aqueous solutions and/or body fluids (blood serum, interstitial fluid) that may induce biotoxicity, if their concentration exceeds the tolerable level. Data on the biodegradation rate of ZnO available in the literature are scarce and controversial. Some reported studies56,57 show very low dissolution rates of ZnO nanostructures at biological pH values (∼7.4), similar to those of bulk ZnO. Fulati et al.13 have found that ZnO nanotubes and nanorods are relatively stable in a buffer solution at a neutral pH ∼ 7. However, other studies report substantial biodegradation rates of nanostructured ZnO. Zhou et al.58 reported that ZnO nanostructures can dissolve within few hours in deionized water (pH ≈ 4.5–5.0), ammonia (pH ≈ 7.0–7.1, 8.7–9.0), and NaOH solution (pH ≈ 7.0–7.1, 8.7–9.0). A study of ZnO nanowires in horse blood serum (pH ≈ 9.0) showed that the ZnO nanowires remain intact in the fluid for only a few hours before they eventually degrade into mineral ions.58 Similarly, Yang and Xie59 demonstrated faster release rates of Zn2+ ions from zinc and zinc oxide nanoparticles than those from microparticles. Substantial biodegradation, which amounted up to 80% of the initial material volume, was observed for ZnO nanorods in venous blood (pH ≈ 7.35–7.45) at 30°C within 10 hours.60 Furthermore, nano-sized materials may exhibit not only higher dissolution rates but also higher solubility limits as compared to their macroscopic counterparts.61 Therefore, both the dissolution rate and solubility of the nanostructured ZnO must be established and taken into account when designing devices using this material system for biomedical applications.
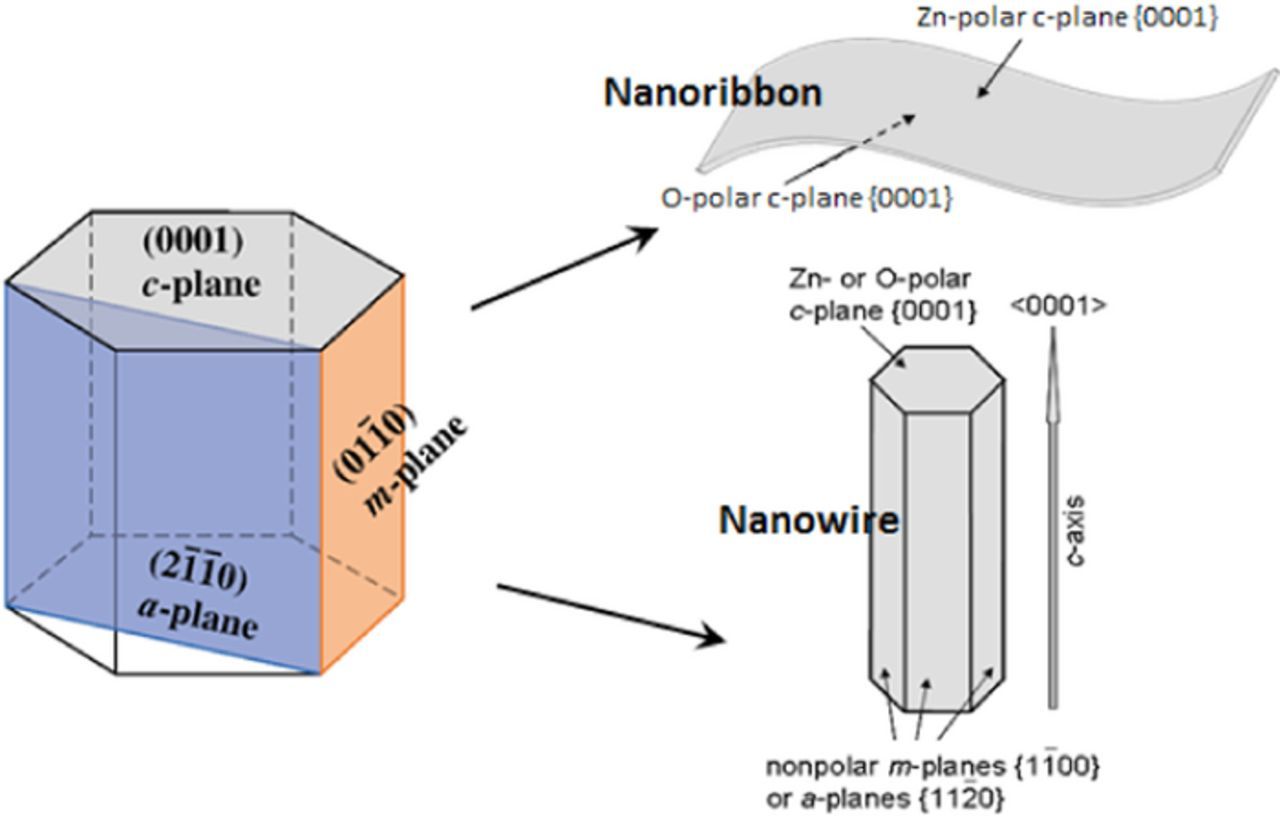
The polar nature of wurtzite ZnO strongly influences the electrochemical properties of different ZnO surfaces through polarization charges, in addition to their different atomic configurations, which leads to different densities of chemisorption sites. ZnO crystals are composed of alternating Zn and O monolayer stacks along the <0001> direction (crystallographic c axis, Fig. 2 right), wherein the valence electron cloud is closer to the O atom, owing to the partial ionicity of Zn - O chemical bonds. This gives rise to positively charged Zn-terminated (Zn-polar) (0001) and negatively charged O-terminated (O-polar)  c-planes, resulting in a difference in surface energy and differing chemical stability of Zn- and O-faces. Nonpolar surfaces (parallel to the <0001> direction) have no polarization charge (Fig. 2) due the equal distribution of Zn and O atoms. Higher wet-etching rates of O-polar compared to Zn-polar surfaces have been reported for ZnO bulk crystals,62,63 albeit lacking quantitative investigations for a wide range of pH values. In alkaline solutions, nonpolar
c-planes, resulting in a difference in surface energy and differing chemical stability of Zn- and O-faces. Nonpolar surfaces (parallel to the <0001> direction) have no polarization charge (Fig. 2) due the equal distribution of Zn and O atoms. Higher wet-etching rates of O-polar compared to Zn-polar surfaces have been reported for ZnO bulk crystals,62,63 albeit lacking quantitative investigations for a wide range of pH values. In alkaline solutions, nonpolar  surfaces have been reported to exhibit relatively high etching rates, comparable to that of the
surfaces have been reported to exhibit relatively high etching rates, comparable to that of the  surface.64 Unfortunately, the published data are very limited.
surface.64 Unfortunately, the published data are very limited.
Figure 2. Polar and nonpolar planes in wurtzite structure (left panel): polar plane is (0001) and nonpolar planes are ( ) (a-plane) and (
) (a-plane) and ( ) (m-plane), which are parallel to the [0001] direction. (Upper right) Schematic sketch of a ZnO nanoribbon terminated by Zn-polar (top face) and O-polar (bottom face). (Bottom right) nanowire grown in polar <0001> direction.
) (m-plane), which are parallel to the [0001] direction. (Upper right) Schematic sketch of a ZnO nanoribbon terminated by Zn-polar (top face) and O-polar (bottom face). (Bottom right) nanowire grown in polar <0001> direction.
Compared to ZnO thin films and bulk crystals, even less is known about the effects of surface orientations on the dissolution rates of nanostructured ZnO. Depending on the growth conditions, the surfaces of ZnO nanostructures can be terminated by Zn- and O-polar c-planes, nonpolar m-planes { } and a-planes
} and a-planes  (Fig. 2), which is responsible, in combination with nanoscale effects, for the observed complex dissolution behavior of nanostructured ZnO.57,65 Moreover, a strong effect of piezoelectric potential generated by strain in bent ZnO nanowires on their dissolving behavior in acidic and alkaline environments has been demonstrated.66 For example, Illy et al.67 have demonstrated that selectivity of electrochemical etching of ZnO nanorods terminated by polar c-plane (the exact polarity of c-plane surface was not specified) and nonpolar
(Fig. 2), which is responsible, in combination with nanoscale effects, for the observed complex dissolution behavior of nanostructured ZnO.57,65 Moreover, a strong effect of piezoelectric potential generated by strain in bent ZnO nanowires on their dissolving behavior in acidic and alkaline environments has been demonstrated.66 For example, Illy et al.67 have demonstrated that selectivity of electrochemical etching of ZnO nanorods terminated by polar c-plane (the exact polarity of c-plane surface was not specified) and nonpolar  planes in acidic media (pH ≈ 3–6) is governed by surface stability and diffusion of reactants. Preferential etching of the less stable c-plane surface was observed for large rod diameters ( ⩾ 100 nm), while homogeneous dissolution was observed for smaller rods (100 nm), when the local chemistry was changed due to diffusion limitations of the dissolution reaction as a result of the nanoscale morphology.
planes in acidic media (pH ≈ 3–6) is governed by surface stability and diffusion of reactants. Preferential etching of the less stable c-plane surface was observed for large rod diameters ( ⩾ 100 nm), while homogeneous dissolution was observed for smaller rods (100 nm), when the local chemistry was changed due to diffusion limitations of the dissolution reaction as a result of the nanoscale morphology.
Other factors, such as doping, can also affect the dissolution of ZnO nanostructures. Because electrical transport in ZnO has a strong effect on sensor sensitivity, the control over ZnO conductivity is essential. Doping with metal ions is usually used to tune ZnO conductivity to meet different application requirements.68 For example, iron (Fe) doping has been used to control the electrical conductive type, carrier concentration, and energy band structure.69 It has also been shown that Fe doping can reduce the dissolution rates of ZnO nanostructures.70
Biofunctionalization of ZnO
Attachment of specific biomolecules on ZnO surfaces is highly important for the realization of a biosensor based on molecular recognition. In general, biomolecules can bind the surface via physical adsorption (e.g. van der Waals, electrostatic, physical adsorption) or chemical binding. Equally important is the establishment of a highly stable and well controlled organic/inorganic interface for efficient charge transfer to achieve high and reproducible sensitivity. Enzymes are commonly used for biological recognition of molecules because most chemical reactions in living systems are catalyzed by very specific enzymes, and immobilization strategies for enzymes are important to preserve their biological activity.71 The high isoelectric point (IEP) of ZnO (∼9.572) makes it possible to attach most of enzymes required for monitoring analytes in human biological fluids via electrochemical attraction.
Glucose Biosensors Based on ZnO Nanostructures
The concentration of glucose in blood is used as an indicator of such diseases as diabetes and endocrine metabolic disorder. Electrochemical enzyme-involved biosensors based on nanostructured ZnO are widely employed for intra/extracellular glucose sensing due to their high sensitivity and selectivity as well as simplicity and relatively low cost of fabrication. Glucose oxidase GOx is the generally used analytical enzyme due to its stability and high selectivity.73 Because ZnO has a high IEP of ∼9.5, it is possible to immobilize low-IEP enzymes, such as GOx (IEP ∼ 4.2), by electrostatic adsorption in proper buffer solutions at the neutral pH values (usually between 7.0 and 7.4, which is similar to those in biofluids).74–77 The common strategy to create a GOx-sensitized electrode is applying GOx dissolved in phosphate buffered saline (PBS) to the electrode surface followed by its coating with Nafion to prevent possible enzyme leakage and eliminate foreign interferences. Glucose detection is based on catalytic reaction between glucose and O2 proceeding on the working electrode with attached GOx that yields gluconic acid and hydrogen peroxide (H2O2).21,71,78 H2O2 is then electrochemically reduces to H+ and the electro-oxidation current is detected after application of a suitable potential to the system:
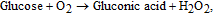
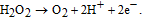
Unfortunately, ascorbic and uric acids and dopamine also can be oxidized under applied voltage, interfering with the glucose-related signal to be monitored. Therefore, selectivity to glucose in the presence of the interfering species is one of the most important figure of merit for biosensors.
As a material for the glucose biosensors, ZnO nanostructures possess numerous advantages including high IEP, biocompatibility, non-toxicity, chemical stability, simplicity of fabrication, large surface area for enzyme immobilization, and abundant space for fluid circulation. Because the degree of enzyme immobilization on the electrode surface depends on the surface area, the morphology of nanostructures has a significant effect on their electrochemical properties.74,79–86 Ahmad et al.84 explored the effect of the aspect ratio of well-aligned ZnO nanorods directly grown on a Si/Ag electrode on glucose oxidase immobilization and resulting sensitivity of biosensors. Of all studied biosensors, the device with the highest aspect ratio of investigated, 60, exhibited the highest sensitivity of 110.76 μA mM−1 cm−2, a very wide linear operation range of 0.01–23.0 mM, and a fast response time of < 1 s. The superior performance of this sensor was attributed to the larger amount of enzymes immobilized on the ZnO nanorods with the highest aspect ratio, i.e. with the highest surface area.
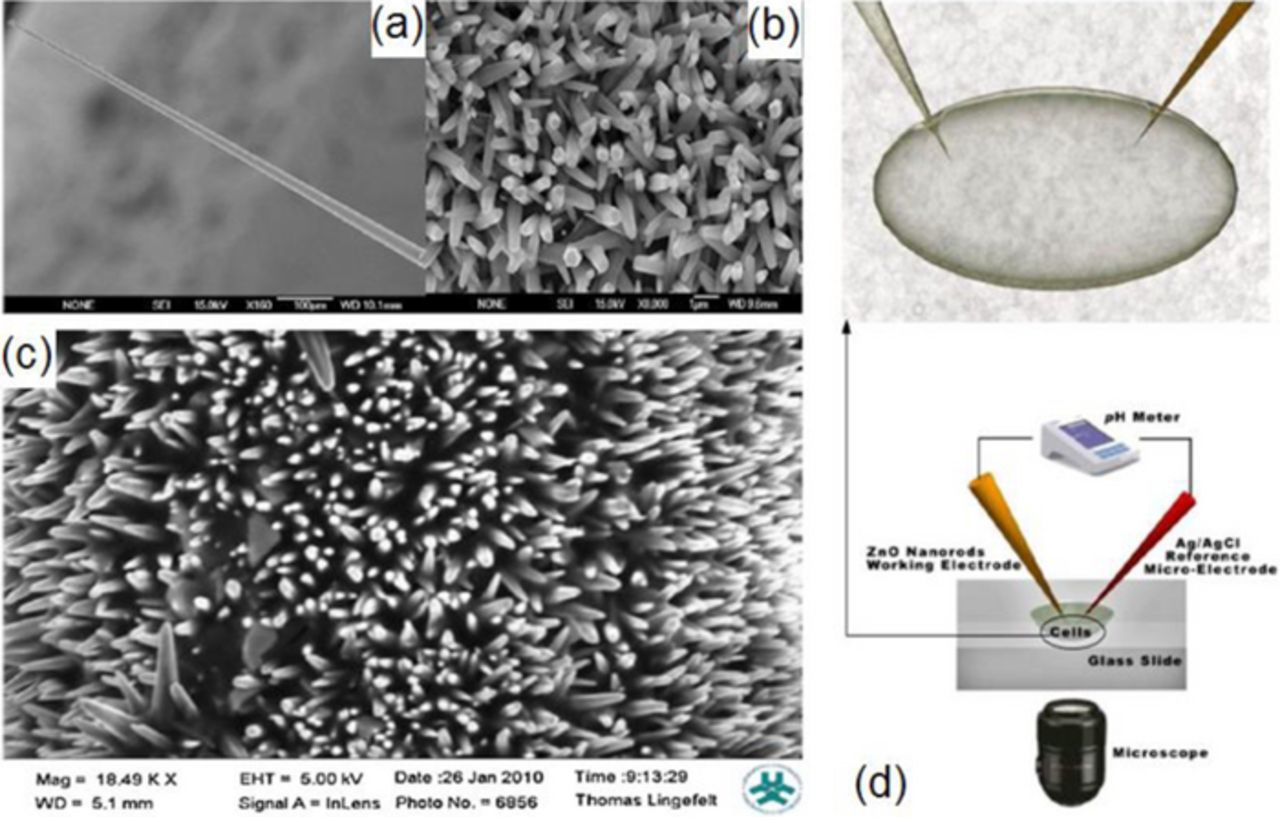
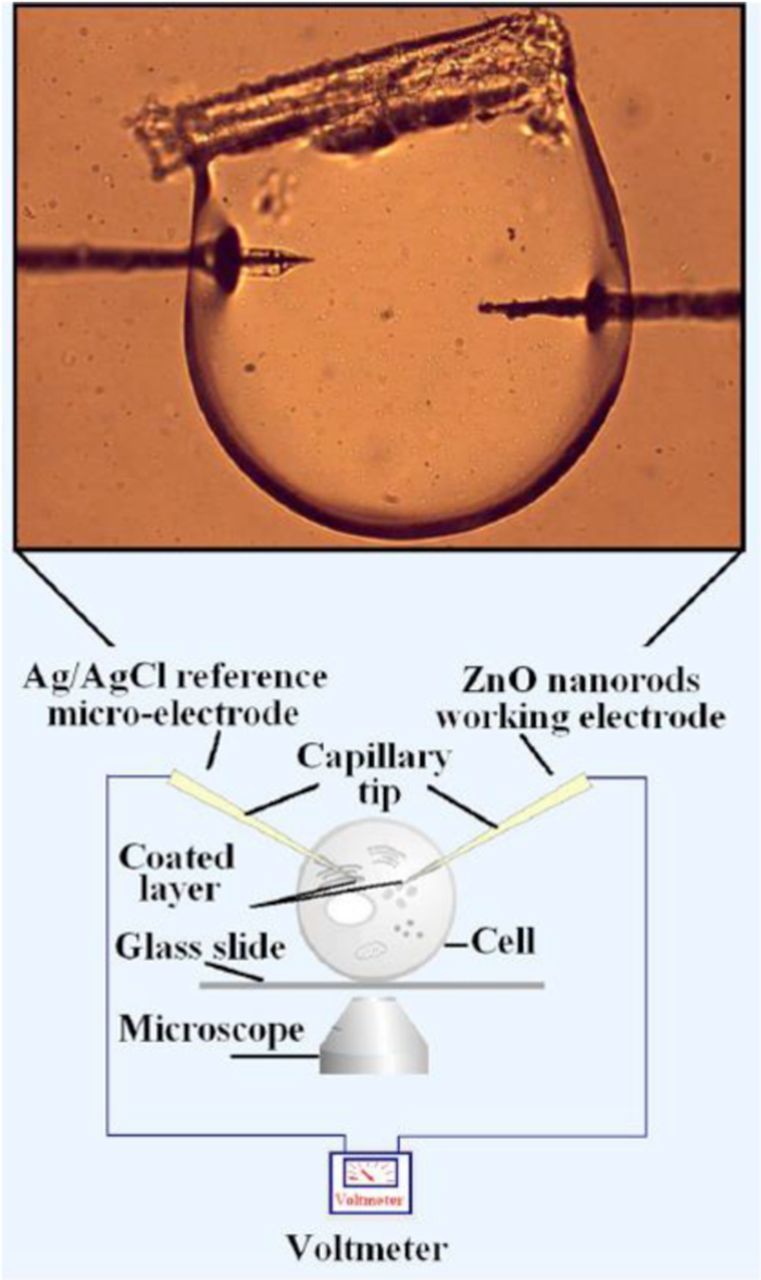
In a somewhat different approach, nanostructures were proven to be a useful construct for intracellular measurements54,71,73,78 Asif et al.71,73 demonstrated a ZnO-nanorod-based selective electrochemical sensor for intracellular measurements of glucose. The nanorods were grown on the tip of silver-covered borosilicate glass capillary 0.7 μm in diameter (Figs. 3a and 3b) using aqueous chemical growth and then functionalized with glucose oxidase (Fig. 3c). Figure 3d shows an optical image of a cell and a schematic diagram of the intracellular measurements with the functionalized ZnO nanorod-coated microelectrodes. The sensor showed a fast (<1 s), linear response to glucose in the concentration range from 0.5 to 1000 μM with a sensitivity of 42.5 mV/decade at around 23°C.71
Figure 3. Scanning electron microscopy images of the ZnO nanorods grown on Ag-coated glass capillaries: (a and b) before enzyme immobilization and (c) after enzyme immobilization. Reprinted Ref. 71, with permission from Elsevier. (d) Optical image of the cell and schematic experimental setup for the intracellular potentiometric measurements. From Ref. 73.
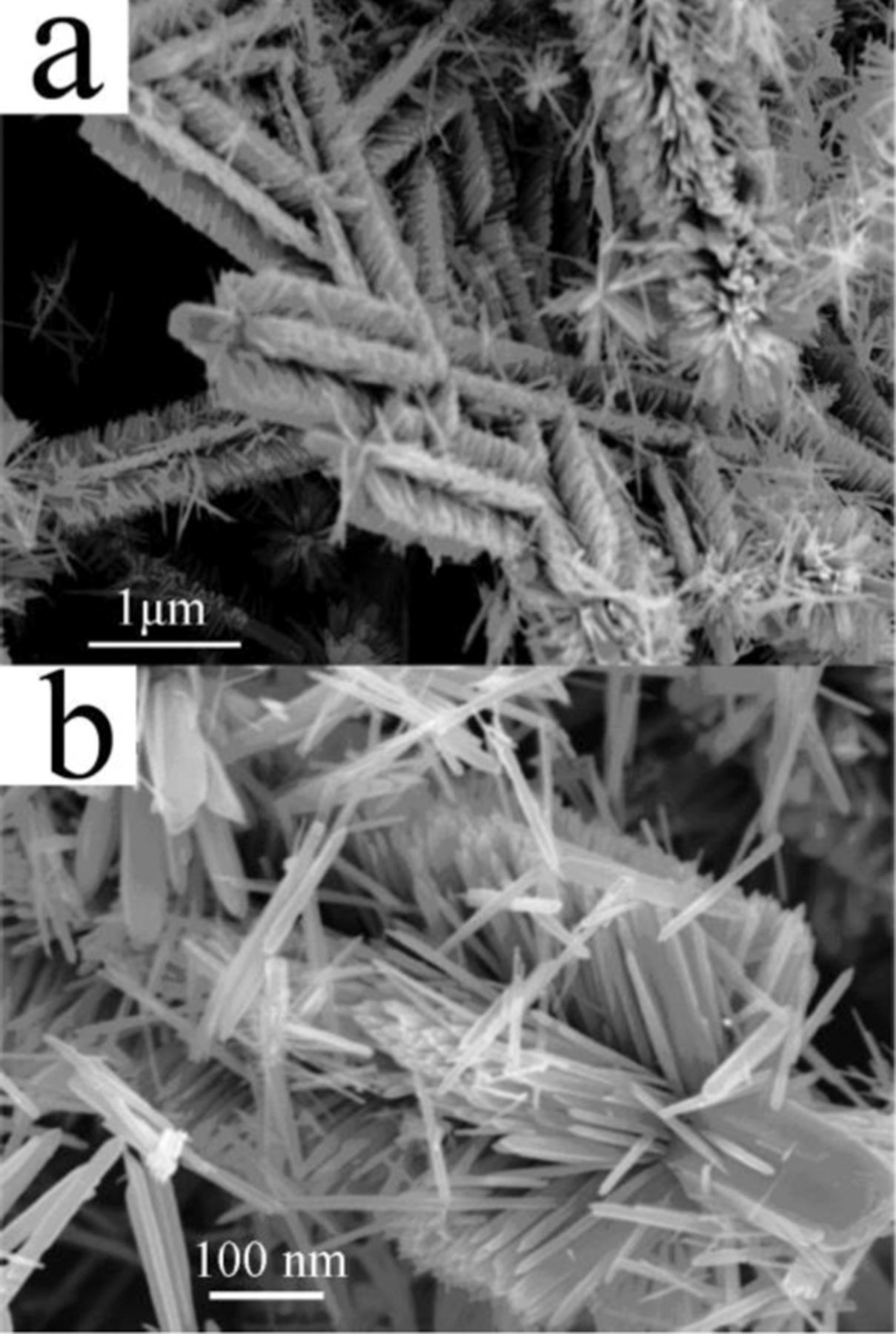
In attempt to increase the surface-to-volume ratio, various hierarchical structures have been studied.80,81,87 Figure 4 shows an excellent example of such a structure, which was prepared by nucleation and growth of smaller nanowires on initial ZnO nanowires.87 Miao et al.81 fabricated a glucose sensor based on ZnO nanowires grown on silicon nanowires by aqueous thermal decomposition. GOx was immobilized on this ZnO/Si hierarchical structure, and a sensor with the Nafion/GOx/ZnO/Si nanowire composite electrode was fabricated. The device showed a high sensitivity of 129 μA mM−1 cm−2 and low detection limit of 12 μM as well as good stability and reproducibility. To test the sensor selectivity, amperometric response was monitored during successive injection of 1.0 mM of glucose, and 10 mM of ascorbic acid, uric acids, and dopamine into 0.1 M PBS (pH = 7.4). The interfering species were found to have a negligible influence, while the response to glucose has been reported to be very strong. Other groups also reported the excellent anti-interference ability of ZnO nanostructure-based sensors to uric and ascorbic acids.21,79,88
Figure 4. Field-emission SEM images of the brush-like hierarchical ZnO nanostructures: (a) at low magnification and (b) at high magnification. Reprinted with permission from Ref. 87. Copyright (2009) American Chemical Society.
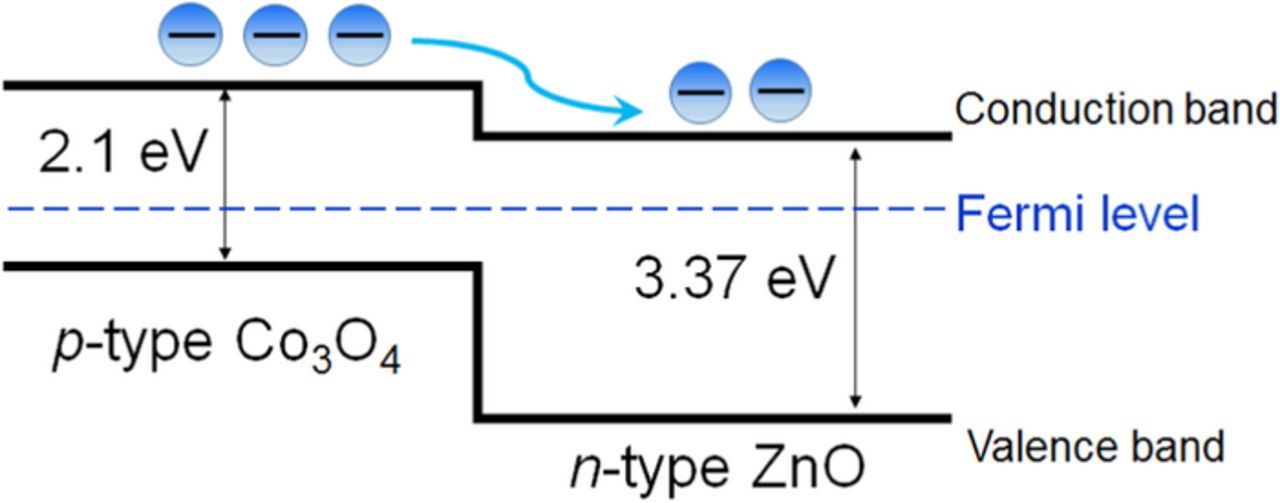
Fan et al.89 proposed to improve the glucose sensing performance by introducing p-n junction interface into the enzyme loading matrix. For this purpose, they fabricated 3D hierarchical Co3O4/ZnO structure by decorating ZnO nanofibers with Co3O4 nanoparticles. Since Co3O4 and ZnO are p- and n-type semiconductors, respectively, p-n junctions are formed at the Co3O4/ZnO interfaces, as shown in Fig. 5. The electric field at the p-n junction interface enhances the electron transfer from the analyst to the electrode. A sensor with the GOx/Co3O4/ZnO working electrode exhibited an enhanced sensitivity of 116.64 μA mM−1 cm−2 as compared to similar sensors with GOx/Co3O4 and GOx/ZnO-modified electrodes (47.55 and 40.57 μA mM−1 cm−2, respectively).
Figure 5. Band diagram of the Co3O4/ZnO p-n junction.
ZnO/metal nanocomposites have also been proposed, constructed, and tested for improved glucose sensing.90–92 In several investigations, it was demonstrated that gold nanoparticles improve immobilization and enzymatic activity of GOx and facilitate direct electron transfer between the redox center of GOx and the electrode surface.93,94 Tian et al.91 fabricated an indium tin oxide (ITO)/ZnO/Au/GOx working electrode by employing a plant-like ZnO nanostructure and chitosan-stabilized spherical Au nanoparticles on which GOx was immobilized. In this study, the ZnO layer was deposited on ITO-coated glass and a layer of Au nanoparticles with an average diameter of 23 nm was coated onto the ZnO surface. The sensor showed a sensitivity of 3.12 μA·mM−1·cm−2 in the linear range of 50–400 mg/dL of glucose. Using ZnO nanorods/Au nanocrystals composite, Wei et al.92 fabricated a glucose sensor with a ZnO/Au//GOx/Nafion-modified working electrode. The sensor showed a linear response to glucose in the concentration rage 0.1 to 33.0 μM, and a sensitivity as high as 1492 μA mM−1 cm−2 was reported.
Non-enzymatic Glucose Sensors
Although the commercial sensors utilizing glucose oxidase enzyme have been on the market for a long time, they suffer from such drawbacks as poor reproducibility and long-term stability of the enzyme. In addition, glucose oxidase is susceptible to ambient conditions, including temperature, pH, and chemical composition of a solution. Irreversible thermal inactivation of GOx starts at 50°C.95 To overcome these problems, a considerable level of effort has concentrated on the search for a stable and inexpensive non-enzymatic electrochemical sensor. The enzyme-free sensors operate on the principle of direct oxidation of glucose on an electrode surface modified with an inorganic electrocatalyst material. Noble (Au, Ag, Pt, Pd) and transition metals (Ni, Cu, Co), composite alloys (Pt-Au, Ni-Cu, Pt-Pb), graphene, carbon nanotubes, polymers, and metal oxides (ZnO, CuO, SiO2, MnO2, TiO2, CeO2, ZrO2,), have been widely studies as catalysts for non-enzymatic glucose sensors.96,97
Here, we discuss the efforts made for the development of electrochemical non-enzymatic glucose sensors based on ZnO nanostructures. Although the glucose sensing ability of ZnO itself was found to be insufficient,98–100 the combination of catalyst species with ZnO nanostructures allows fabrication of hierarchical structures with a large surface area providing large area for catalysis, short diffusion pathways for charge carriers, and efficient electron transport, thus improving sensor performance. Various catalyst species, including noble metals Au98 and Pt,99 Ni101 and its compounds,102–104 CuO,105–109 and carbon nanotubes,110 have been studied for fabricating non-enzymatic glucose sensors based on ZnO nanostructures (see Table II).
Table II. Comparison of glucose biosensors based on ZnO nanostructures.
| Nafion/GOx/ZnO nanorods/Ag/Si substrate | 110.76 μA mM−1 cm−2 | 0.01–23.0 mM | 0.1 μM | < 1 s | 0.055 mM | 45 days / 94% | 84 |
| Single ZnO nanofiber on Au electrode | 70.2 μA mM−1 cm−2 | 0.25–19 mM | 1.0 μM | ∼4 s | 2.19 mM | 120 days / 95% | 86 |
| Nafion/GOx/ZnO nanowires /Si nanowires /Si wafer | 129 μA mM−1 cm−2 | 0.2–20 mM | 12 μM | 5 weeks / 92% | 81 | ||
| EGFET based on Al-doped ZnO nanostructures | 60.5 μA mM−1 cm−2 | 0–13.9 mM | 83 | ||||
| Nafion/GOx/ZnO nanotubes /ITO/glass | 30.85 μA mM−1 cm−2 | 10 μM–4.2 mM | 10 μM | <6 s | 2.59 mM | 77 | |
| Nafion/GOx/Au NPs/plant-like ZnO/ITO/glass | 3.12 μA mM−1 cm−2 | 50–400 mg/dL | 1.70 mM | 91 | |||
| GOx/Au NPs/3D hierarchical ZnO nanostructures/GCE | 1.409 μA mM−1 | 1–20 mM | 20 μM | 15 days / 94.4% | 90 | ||
| Nafion/GOx/Au nanocrystals/ZnO nanorods/GCE | 1492 μA mM−1 cm−2 | 0.1–33.0 μM | 10 nM | 5 s | 0.41 mM | 3 months / 90% | 92 |
| Chitosan/GOx/Co3O4-ZnO nanostructures/GCE | 116.64 μA mM−1 cm−2 | 0.01–5 mM | 1.38 μM | 4 s | 3 weeks / 91% | 89 | |
| GOx/ZnO nanorods/borosilicate glass capillary | 42.5 mV/decade | 500 nM–1 mM | < 1 s | 71 | |||
| Non-enzymatic | |||||||
| Zn0.90Co0.10O nanoparticles/GCE | 5 μM | <4 s | 1 month | 100 | |||
| ZnO/MWCNTs/GCE | 64.29 μA mM−1 cm−2 | 1–10 mM | 0.82 mM | 1 month / ∼100% | 110 | ||
| Ni(OH)2 nanorods/ZnO nanorods/ITO | 1569 μA mM−1cm−2 | 2–3862 μM | 0.6 μM | < 10 s | 102 | ||
| Ni nanoparticles/ ZnO hexagonal prisms/GCE | 824.34 μA mM−1 cm−2 | 1 μM–8.1 mM | 0.28 μM | 4 s | 1 month / 90.2% | 101 | |
| Ni(OH)2 nanoflakes/ZnO nanorods/ITO | 1.85 mA mM−1 cm−2 | 0.04–2.10 mM | <1 s | 40 days / 95% | 103 | ||
| NiO/ZnO mesoporous composite/GCE | 120.5 μA mM−1 cm−2 | 0.5 μM–6.4 mM | 0.5 μM | <3 s | 104 | ||
| ZnO–CuO hierarchical nanocomposites/FTO/glass | 3066.4 μA mM−1 cm−2 | 0.47 μM–1.6 mM | 0.21 μM | 3 weeks / 93% | 105 | ||
| ZnO–CuO porous spheres core–shell structure composite with/GCE | 1,217.4 μA mM−1 cm−2 | 0.02–4.86 mM | 1.677 μM | <2 s | 106 | ||
| ZnO nanorods/CuO nanoleafs/Cu substrate | 408 μA mM−1 cm−2 | 0.1–1 mM | 18 μM | 107 | |||
| CuO-ZnO composite nanofibers/Pt | 463.7 μA mM−1 cm−2 | 8.00 × 10−7–3.88 × 10−3 M | 0.126 μM | 1 month / 90% | 108 | ||
| CuO nanoflowers/ZnO nanorods/brass substrate | 1362.7 μA mM−1 cm−2 | 1 μM | 5 s | 109 | |||
Among the potential catalytic materials, Pt and Au are considered to be less promising. In addition to the high cost of the precious metals, Pt degrades in the presence of some species commonly present in human serum, such as chloride anions, uric and ascorbic acids, amino acids, and acetaminophen.97 Gold also suffers from chloride poisoning and poor chemisorption of glucose on Au electrodes.97 On the other hand, non-precious transition metals, including Ni, Cu, and Co, are much cheaper and do not suffer from chloride poisoning. Vijayaprasath et al.100 studied non-enzymatic glucose biosensor based on Co-doped ZnO nanostructures. Pure ZnO and Zn1-xCoxO (x = 0.05, 0.10 and 0.15) nanoparticles were synthesized by co-precipitation method and used for fabrication of electrochemical glucose biosensor. The cyclic voltammetric behavior of electrodes modified with pure ZnO, Zn0.85Co0.15O, Zn0.90Co0.10O, and Zn0.95Co0.05O nanoparticles in natural pH (7.4) medium, 0.01 M PBS, in the presence of 1 mM glucose was studied. While the pure ZnO-modified electrode showed no significant current change, the electrodes modified with Zn1-xCoxO nanoparticles showed enhanced oxidation current, with the best results obtained for Zn0.90Co0.10O sample, which was attributed to its highest porosity. The amperometric response of the Zn0.90Co0.10O -modified electrode was examined in 0.01 M PBS by measuring the catalytic current at a potential of 0.4 V with successive addition of 1 mM glucose. The electrode responded quickly to the addition of glucose (< 4 s), with a detection limit of 5 μM. No effect of interfering substances, including L-dopa, ascorbic acid, hydrogen peroxide and uric acid (1 mM), on the biosensor response was detected.
Ni and its compounds exhibit very high catalytic activity in alkaline media due to the Ni3+/Ni2+ redox couple.96 In an alkaline medium, Ni is oxidized into Ni(OH)2 (Ni2+) and then further oxidized into NiOOH (Ni3+), the compound which works as a catalyst for the oxidation of glucose to gluconolactone:
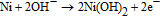
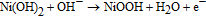
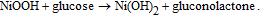
Enzyme-free glucose sensors based on Ni-ZnO,101 Ni(OH)2-ZnO,102,103 or NiO-ZnO104 nanocomposites have been recently demonstrated. Yang et al.102 have fabricated a non-enzymatic glucose sensor based on hollow Ni(OH)2 nanorods deposited on ZnO nanorods via chemical bath deposition. The Ni(OH)2-based sensor was tested in 0.1 M NaOH solution (pH = 13) and exhibited a high sensitivity of 1569 μA mM−1cm−2 over a wide linear detection range of 2 to 3862 μM with a detection limit of 0.6 μM. The device showed excellent selectivity in the presence of L-aspartic acid, uric acid, dopamine, and ascorbic acid, which proves it can be used to detect glucose in human blood serum. Moreover, the sensor can function at temperatures as high as 75°C with an associated sensitivity of 2904.9 μA mM−1cm−2.
Similar to Ni and its compounds, CuO can serve as electrocatalyst for glucose oxidation in the alkaline electrolyte.105–108 In this case, the catalytic reaction is mediated by the Cu2+/Cu3+ redox couple. The process can be described as follows:
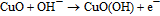
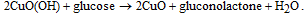
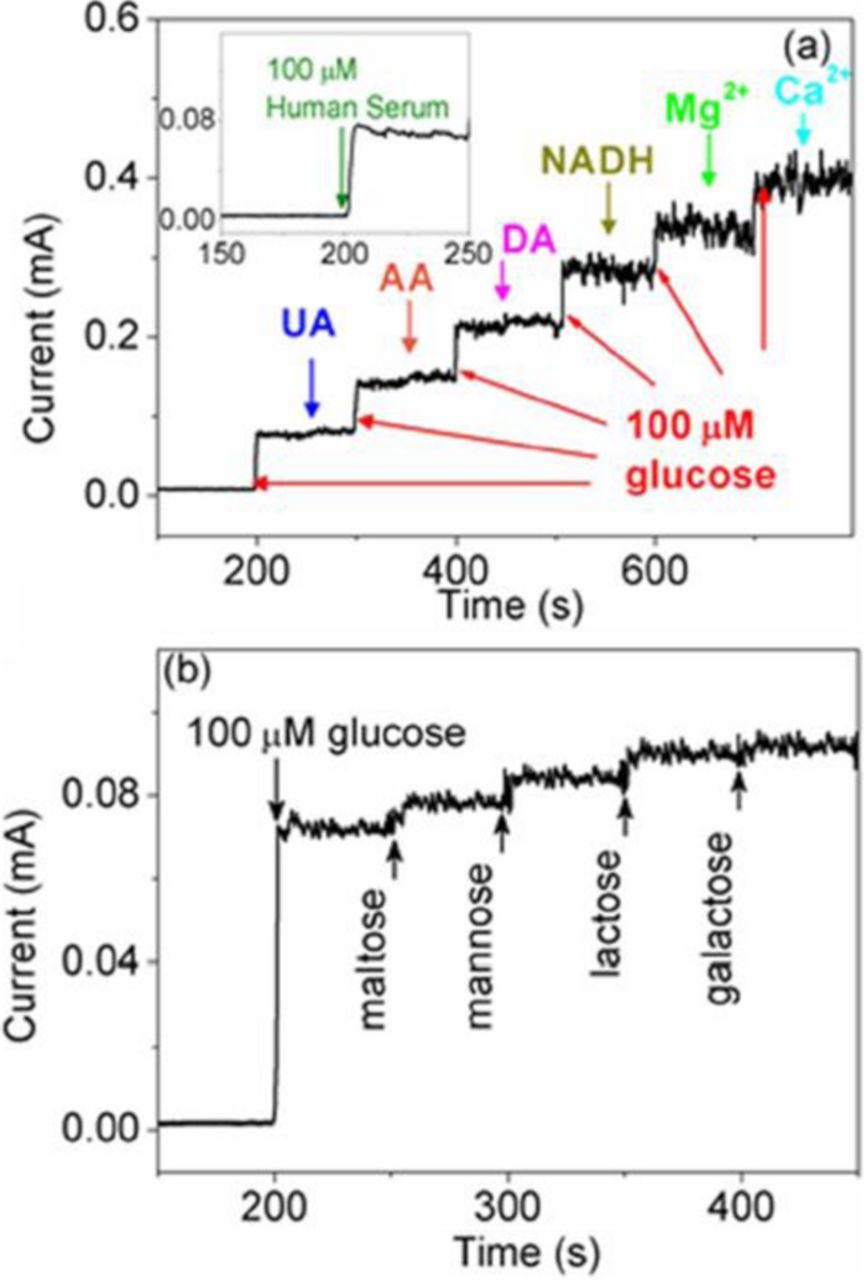
The oxidation–reduction reaction is accompanied by transfer electrons. Due to the favorable energy-band structure at the ZnO/CuO hetero-junction, the electron transfer from CuO to ZnO is facilitated, thus improving the glucose sensing performance.106,109 The CuO/ZnO-modified electrodes showed good long-term stability (within weeks),100–103,105,108,110 reproducibility, and selectivity against interfering species, including uric acid, dopamine, ascorbic acid, nicotinamide adenine dinucleotide (NADH), Mg2+ and Ca2+ ions, normally present in human blood.105–108 Figure 6a shows the amperometric response of the porous ZnO–CuO hierarchical nanocomposite/fluorine-doped tin oxide (FTO) electrode the interfering species measured by Zhou et al.105 As compared to glucose, these species have almost no effect on the current. Addition of sugars (lactose, mannose, galactose, maltose) result in more pronounced response (see Fig. 6b), albeit lower than the response to glucose. Moreover, the sensor was tested in human blood serum, and an obvious current response was observed after addition of 100 mM glucose, as shown in inset of Fig. 6a. The glucose concentrations measured by the CuO/ZnO-based sensor were close to those obtained with the Accu-Chek active glucose meter, indicating the potential of the proposed non-enzymatic sensor for real sample analysis.
Figure 6. Amperometric response of 3D porous ZnO–CuO hierarchical nanocomposites electrode (a) with successive additions of different interfering species uric acid (UA), ascorbic acid (AA), dopamine (DA), nicotinamide adenine dinucleotide (NADH), Mg2+ and Ca2+ ions and (b) with sequential addition of 10 μM various interfering sugars (maltose, mannose, lactose, and galactose) after initial addition of 100 μM glucose. The inset of (a) is current response toward to 100 mM glucose in human serum. Glucose to interfering species ratio is 10:1. From Ref. 105.
It should be mentioned here that enzymatic sensors rely on highly selective catalytic enhancement of oxidation reaction of a target analyte, which gives rise to high selectivity with respect to interfering species (e.g. glucose vs. dopamine, ascorbic acid, uric acid etc.). Therefore, it is worth discussing here how ZnO-based sensors can be selective in the absence of the enzyme. The good selectivity of ZnO-CuO, ZnO-NiO, and ZnO-Ni(OH)2-modified electrodes against some electroactive species, such as ascorbic acid, L-aspartic acid, dopamine acid, and uric acid, have been explained by electrostatic repulsion.102,103,105,111 Under highly basic conditions in 0.1 M NaOH solution, ZnO, CuO, NiO, and Ni(OH)2 having high isoelectric points are negatively charged, and the interfering species (ascorbic acid, L-aspartic acid, dopamine, uric acid) are also negatively charged due to the loss of a proton. As a result, the interfering species are repelled from the surface of the modified electrode and their electrochemical oxidation is prevented. It should be noted, however, that biosensors designed for biomedical application should provide high selectivity at pH values close to 7.4 which are characteristic for biofluids. It is also should be pointed out that, in selectivity tests of the non-enzymatic sensors, the ratio of glucose to interfering species is usually 10 : 1, considering that the glucose concentration in normal human blood is at least 30 times higher than those of the common interfering species.105,107,108 As an example, Fig. 6b indicates that, in addition to a pronounced response to 100 μM glucose, a moderate increase in current is observed upon sequential addition of 10 μM of various interfering sugars (maltose, mannose, lactose, and galactose). We may expect that the interfering sugars could produce the oxidation current comparable to that of glucose if their concentrations were the same as glucose concentration. Thus, the non-enzymatic sensors rely mainly on expected low concentration of interfering species in human blood, rather than on catalytic activity specifically to glucose. Another drawback of the non-enzymatic sensors is that the glucose detecting reactions involve OH– anions, so the sensors are operative only in alkaline solutions, and, consequently, blood samples should be diluted with alkaline electrolytes for the measurements.96
Cholesterol Biosensors Based on ZnO Nanostructures
Cholesterol is one of the main components of the human body. The normal concentration of cholesterol in human serum is below 5.2 mM (200 mg dL−1), while anomalous levels of cholesterol in blood cause various clinical disorders, such as heart disease, coronary artery disease, cerebral thrombosis, arteriosclerosis, hypertension, lipid metabolism dysfunction, arteriosclerosis, and myocardial infarction.112,113 For cholesterol detection in electrochemical biosensors, cholesterol oxidase, ChOx, (IEP ≈ 4.7) is often used to sensitize the ZnO surface. As in the case of GOx, immobilization occurs via physical adsorption due to electrostatic attraction. ChOx catalyzes the reaction between cholesterol and oxygene to produce hydrogen peroxide. The enzymatic reaction of ChOx is as follows:
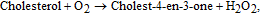
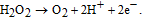
As in the case of ZnO based glucose sensors, to increase the amount of immobilized enzyme, various ZnO nanostructures, including nanoparticles,114 nanospheres,23 nanowalls,115 nanotubes,116 and nanorods,117,118 have been employed for cholesterol sensor fabrication (see Table III). A highly sensitive amperometric cholesterol biosensor based on platinum-incorporated fullerene-like ZnO hybrid nanospheres (50–200 nm) has been fabricated by electrodeposition of nanospheres onto a glassy carbon electrode (GCE).23 The enzyme solution was prepared by dissolving ChOx (1.0 mg/mL) in 0.1 M (0.9% NaCl) PBS (pH 6.8) for 24 h and then immobilized on the surface of as-prepared ZnO nanospheres via physical adsorption. After drying the modified electrodes in air for 2 h, a Nafion solution was dropped onto the electrode surfaces. The resulting biosensor exhibited a sensitivity of 1886.4 mA/(M cm2) featuring a linear range from 0.5 to 15 μM and a detection limit of less 0.2 μM, which resulted from increased enzyme activity. It has been found that the combination of ZnO and platinum nanoparticles facilitates low potential (0.2 V) amperometric detection of cholesterol with high detection selectivity. Wang et al.117 reported a biosensor based on Pt/Au hybrid-functionalized ZnO nanorods, having a detection limit of 0.03 μM with high selectivity for ascorbic acid, uric acid, and L-cystine. The sensor was applied to the determination of cholesterol in diluted blood serum samples with satisfactory results.
Table III. Comparison of cholesterol biosensors based on ZnO nanostructures.
| Electrode | Sensitivity | Linear range | Detection limit | Response time | Apparent Michaelis-Menten constant | Long-term stability / % of original response | Ref. |
|---|---|---|---|---|---|---|---|
| Stabilized lipid film/ChOx/ZnO nanowalls/Al foil | 57 mV/ decade | 10−6–10−3 M | 4 × 10−7 | ∼5 s | >4 weeks / 90% | 115 | |
| Nafion/ChOx/Pt-incorporated fullerene-like ZnO nanospheres/GCE | 1886 mA/M−1 cm−2 | 0.5–15 μM | <0.2 μM | <5 s | 30 days / 95% | 23 | |
| Nafion/ChOx/ZnO nanorods/Ag/Si substrate | 74.1 μA mM−1 cm−2 | 0.01–16.0 mM | 0.0015 μM | <2 s | 0.16 mM | 45 days / 95% | 119 |
| Nafion/ ChOx/Pt-Au-functionalized ZnO nanorods /chitosan-MWCNTs/GCE | 26.8 μA mM−1 | 0.1–759.3 μM | 0.03 μM | <6 s | 1.84 mM | 4 weeks / 82.5% | 117 |
| Nafion/ChOx/ZnO nanoparticles/Au | 23.7 μA mM−1 cm−2 | 1.0–500.0 nM | 0.37 nM | < 5 s | ∼4.7 mM | 50 days / 91.8% | 114 |
| Nafion/ChOx/ZnO nanorods/Au | 61.7 μA μM−1 cm−2 | 1.0–15.0 μM | 0.012 μM | <5 s | 2.57 mM | 32 days / 83.7% | 118 |
| ChOx/nano-ZnO/ITO | 0.059 μA/mg dl−1 cm−2 | 5–400 mg/dl | 0.5 mg/dl | 10 s | 0.98 mg/dl | 3 months / 80% | 122 |
| ChOx/nano-structured ZnO/Pt/Si | 153 μA mM−1 cm−2 | 0.12–12.93 mM | 5 s | 1.08 mM | >10 weeks / >90% | 120 | |
| ChEt–ChOx/ZnO/Pt/Si | 117 μA mM−1 cm−2 | 0.5–12 mM | 0.98 mM | 121 | |||
| ChOx/ZnO nanocrystals/Ag nanowires/graphene oxide- chitosan/ITO | 9.2 μA μM−1 cm−2 | 6.5 μM–10 mM | 0.287 μM | 0.295 μM | 30 days / 97.9% | 123 | |
| Nafion/ChOx/ZnO nanotubes/Ag/Si | 79.40 μA mM−1 cm2 | 1 μM–12 mM | 0.5 nM | ∼2 s | 0.25 mM | 60 days / 93% | 116 |
| ChOx/ZnO-ZnS nano-heterostructure/ chitosan/GCE | 293 mA M−1 cm−2 | 0.4–2 mM | 0.08 mM | <5 s | 0.547 mM | 30 days / 93–95% | 22 |
| ChOx/ chitosan/ZnO-ZnS microtubes/GCE | 52.67 μA mM−1 cm−2 | 0.4–3.0 mM | 0.02 mM | <5 s | 30 days / 95% | 124 |
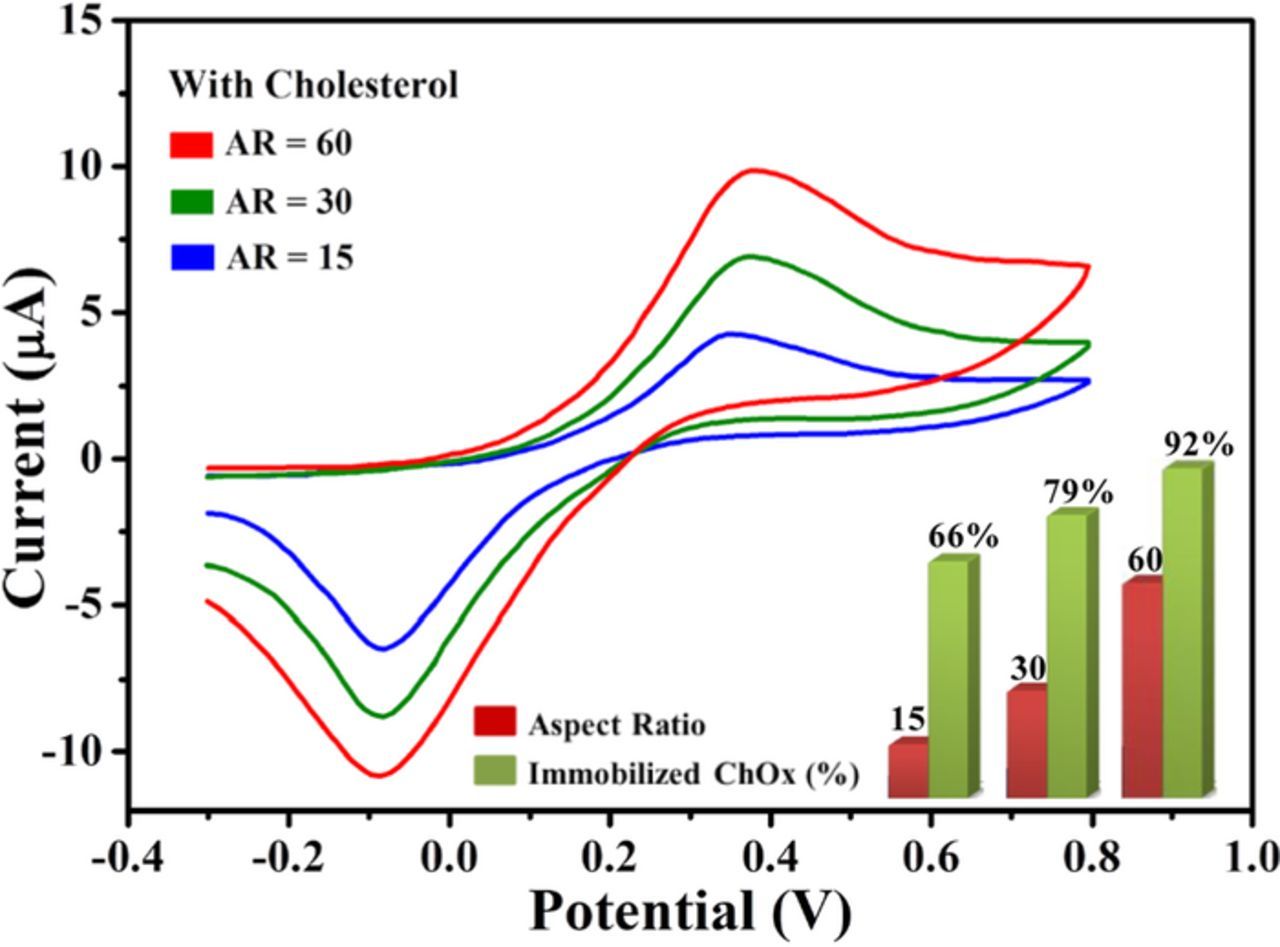
Ahmad et al.119 studied the effect of aspect ratio of ZnO nanorods grown directly on a Ag electrodes on the performance of cholesterol biosensors. As in the case of glucose sensing,84 they have found that an increase in the aspect ratio leads to a larger enzyme load and enhanced electron conduction, which results in better sensitivity of the sensors, as illustrated in Fig. 7. One can see in the figure that the amount of immobilized ChOx increases as the aspect ratio increases, resulting in improved performance of the ZnO-modified electrode.
Figure 7. Cyclic voltammetry sweep curves for the aspect-ratio dependent biosensors in the presence of 2 mM cholesterol in 0.1 M PBS (pH = 7.4). Aspect ratio = 15 (blue line), 30 (green line) and 60 (red line) in the range of −0.3 to +0.80 V at scan rate of 100 mV/s. Also shown are histograms presenting the relationship between the enzyme immobilization percentage and the aspect ratio of ZnO nanorods. Courtesy of Prof. Yoon-Bong Hahn.
Psychoyios et al.115 fabricated a working electrode based on ZnO nanowalls encapsulated with a ChOx-containing lipid membrane. The potentiometric biosensor showed an extremely wide logarithmic detection range of 10−6 M to 103 M with a sensitivity slope curve of ∼57 mV/decade and high selectivity for a variety of analytes (maltodextrin, dextrose, fructofuranose, ascorbic acid, lactose, sorbitol, glucose, leucine, etc.). The excellent sensing characteristics of functionalized ZnO nanowalls were attributed to their large surface area and ease in electrical communication. To evaluate the ability of the biosensor for routine analysis, it was tested in diluted blood serum samples. The measured cholesterol concentrations were in good agreement with those measured by a hospital-grade biochemical analyzer, which underscores the reliability of the biosensor for cholesterol level determination in real samples.115 An ultra-low detection limit of 0.37 ± 0.02 nM was achieved for a cholesterol biosensor prepared using ZnO nanoparticles by solution growth at low-temperature on a gold electrode.114
Nanostructured ZnO layers were synthesized by vapor phase transport deposition on Pt coated Si, and then loaded with ChOx120,121 plus another enzyme, cholesterol esterase (ChEt).121 The resulting high sensitivities (153 μA/(mM cm2)120 and 117 μA/(mM cm2)121 for ChOx/ZnO/Pt/Si and (ChEt-ChOx)/ZnO/Pt/Si, respectively) of the biosensors for detection of free cholesterol (0.12–12.93 nM) and total cholesterol (0.5–12 mM) is indicative of successful immobilization of enzymes via physical absorption and good transport properties of the structure. Solanki et al.122 also reported cholesterol biosensors prepared by ChOx immobilization on nanostructured ZnO films fabricated by sol–gel process on ITO. These sensors exhibited a wide linear range of 5.0–400 mg/dL, a high sensitivity of 59 nA/(mg dL cm2), and a low detection limit of 0.5 mg/dL.
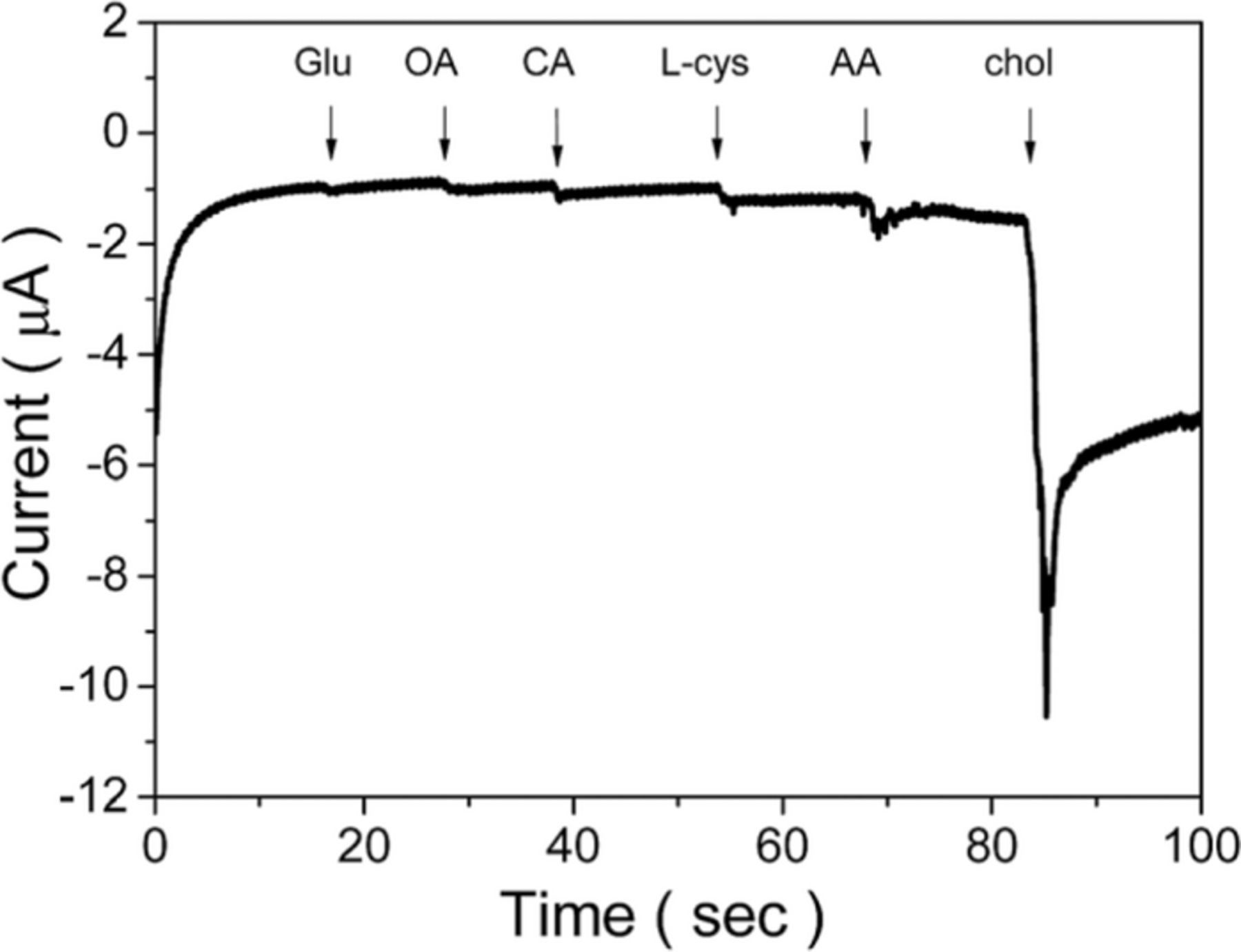
Wu et al.123 have fabricated a highly sensitive and selective amperometric cholesterol biosensor based on an Ag/ZnO nanocomposite immobilized on graphene oxide-chitosan/ITO electrode. Here, Ag nanowires were used to catalyze the reduction of H2O2, ZnO nanocrystals helped to immobilize ChOx (IEP ∼ 5.0) via strong electrostatic binding. The modified ChOx/ZnO/Ag/graphene oxide-chitosan/ITO working electrode was tested in 10 mM PBS and a 200 mg dL−1 cholesterol solution (pH = 7.0) with a platinum coil counter electrode and an Ag/AgCl reference electrode. The sensor exhibited a high sensitivity of 9.2 μA μM−1 cm−2 in a linear range from 6.5 mM to 10 mM. The apparent Michaelis–Menten constant was calculated to be as low as 0.295 μM, indicating the high affinity of the ChOx/ZnO/Ag/graphene oxide–chitosan/ITO electrode to cholesterol. The electrode showed also good stability, the measured response decreased by 2.1% in 10 days, and 4.7% in 30 days, and excellent selectivity to glucose, oxalic acid, citric acid, L-cysteine, and ascorbic acid (Fig. 8). No pronounced current increment was observed although the amounts of the interfering species were up to 20 times greater than cholesterol concentration (5.2 mM). The ChOx/ZnO/Ag/graphene oxide-chitosan/ITO electrode was tested also in real samples with acceptable recoveries (98.3–102.3%).
Figure 8. Amperometric response of the interference test for the ChOx/ZnO/Ag/graphene oxide–chitosan/ITO electrode in phosphate buffer (pH 7.0) at a potential of −0.50 V with the addition of 0.1 M glucose (Glu), oxalic acid (OA), citric acid (CA), L-cysteine (L-Cys), and ascorbic acid (AA). Reproduced from Ref. 123 with permission of The Royal Society of Chemistry.
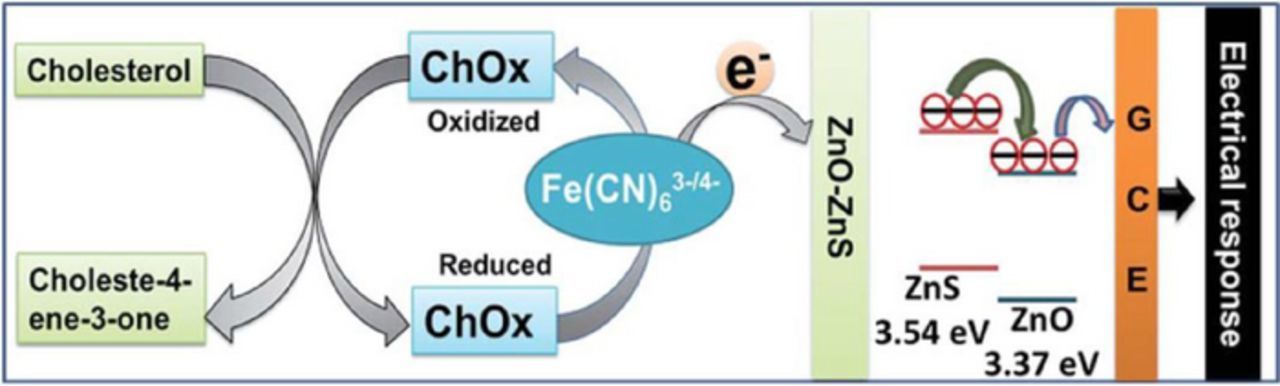
Giri et al.22,124 made an attempt to improve the sensing ability by using ZnS-ZnO heterostructures. They fabricated ChOx/ZnS-ZnO heterostructure-modified electrodes, where electric field at the ZnS/ZnO interface was supposed to facilitate electron transfer, thus increasing sensor performance. As shown in Fig. 9, the conduction band of zinc blend ZnS lies higher than that of wurtzite ZnO, so that electrons, generated by ChOx are transferred to the outer ZnS via the Fe2+/Fe3+ redox couple as mediator, and the electric field at the ZnS/ZnO interface accelerates electrons moving to the glassy carbon electrode.
Figure 9. Schematic presentation of the probable sensing mechanism of the ZnO-ZnS microtube-based electrode, ChOx/Chitosan/ZnO-ZnS/GCE in PBS (0.05 M, pH 7.0, 1% NaCl) containing [Fe(CN)6]3–/4–. Reproduced from Ref. 124 with permission of The Royal Society of Chemistry.
Umar et al.118 systematically studied the effect of pH level on the performance of cholesterol sensors based on ZnO nanostructures. It was found that the current response reaches its maximum in the pH range of 6.8–7.6 and decreases on either side of this pH range. This finding was attributed to the effect of pH on the affinity of enzymes for a substrate. At low pH values (pH < 7), H+ ions compete with enzyme species for any cationic binding sites at the substrate, thus decreasing enzyme activity. At high pH values (pH > 7), hydroxyl ions may lead the conversion of hydroxides and hinder the enzyme activity, causing the reduction in the sensitivity of the biosensor.
L-lactic Acid Biosensors Based on ZnO Nanostructures
Increased concentration of lactic acid in blood is indicator of various disorders, such as shock, heart failure, chronic renal failure, respiratory insufficiency, chronic renal failure, and metabolic disorders.125 Determination of lactic acid concentration is also useful for sports medicine and the food industry, where lactic acid content serves as an indicator of food freshness. Among four enzymes suitable for L-lactic acid sensing, namely lactate dehydrogenase (LDH), cytochrome b2 (Cyt b2), lactate monooxygenase (LMO), and lactate oxidase (LOD), the latter is most commonly used due to simplicity of its catalytic process.27 Negatively charged LOD (IEP = 4.2–4.5) can be mobilized on ZnO surface through electrostatic interaction. In the presence of immobilized LOD, L-lactic acid is oxidized with formation of unstable pyruvic acid, which immediately decomposes into pyruvate and H2O2. Hydrogen peroxide reduces to H+, and electro-oxidation current is detected at an LOD-sensitized electrode according to the following catalytic reactions:
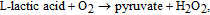
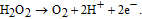
As in the cases discussed above, ZnO nanostructures have also been shown to provide a viable platform for lactic acid biosensing.26,27–128 Wang et al.126 fabricated a multi-layer hybrid component electrode, where LOD was immobilized on ZnO nanoparticles deposited on multi-walled carbon nanotubes (MWCNTs). A polydiallyldimethylammonium chloride (PDDA) was coated on the LOD layer. The unique sandwich-like layer structure of the PDDA/LOD/ZnO/MWCNTs cathode provided a favorable microenvironment to keep the bioactivity of the enzyme and also prevented leakage of the LOD molecules. The sensors were tested in PBS and showed a linear response range of 0.2 to 2.0 mM, a sensitivity of 7.3 μM/mM, and a detection limit of 6 μM, a good reproducibility and selectivity (see Table IV). The response current was found to depend on the pH of PBS, and a maximum response was obtained at pH = 6.8. Thermal stability of LOD immobilized on ZnO nanoparticles was studied in the temperature range from 10 to 50°C in comparison with a sensor fabricated by using Pt electrodes modified with LOD-glutaraldehyde-bovine serum albumin (BSA). The response of the PDDA/LOD/ZnO/MWCNT sensor was found to increase continuously with temperature, exhibiting an Arrhenius temperature dependence, while the response of the LOD–glutaraldehyde–BSA sensor dropped drastically at temperatures above 40°C. The superior thermoresistance of the PDDA/LOD/ZnO/MWCNT sensor was attributed to its multilayer design, where the ZnO nanoparticles provided a favorable environment for the immobilized LOD.
Table IV. Comparison of lactic acid biosensors based on ZnO nanostructures.
| Electrode | Sensitivity | Linear range | Detection limit | Response time | Apparent Michaelis constant Kapp | Life time (% of original bioactivity) | Ref. |
|---|---|---|---|---|---|---|---|
| PDDA/LOD/ZnO nanoparticles/MWCNTs | 7.3 μM mM−1 | 0.2–2.0 mM | 6 μM | 6 s | 1.5 mM | 120 days / 95.1% | 126 |
| Nafion/LOD/ZnO nanotetrapods/Au | 28.0 μA mM−1 cm−2 | 0.0036 –0.6 mM | 1.2 μM | 10 s | 0.58 mM | 27 | |
| LOD/ZnO nanorods/Au/glass | 41.33 mV/decade | 10−4 – 1 mM | 1 × 10−4 mM | <10 s | 3 weeks 98.7% | 26 | |
| LOD/In-doped ZnO nanowires-gated AlGaAs/GaAs HFET | 3 pM–3 mM | 3 pM | 1 s | 131 |
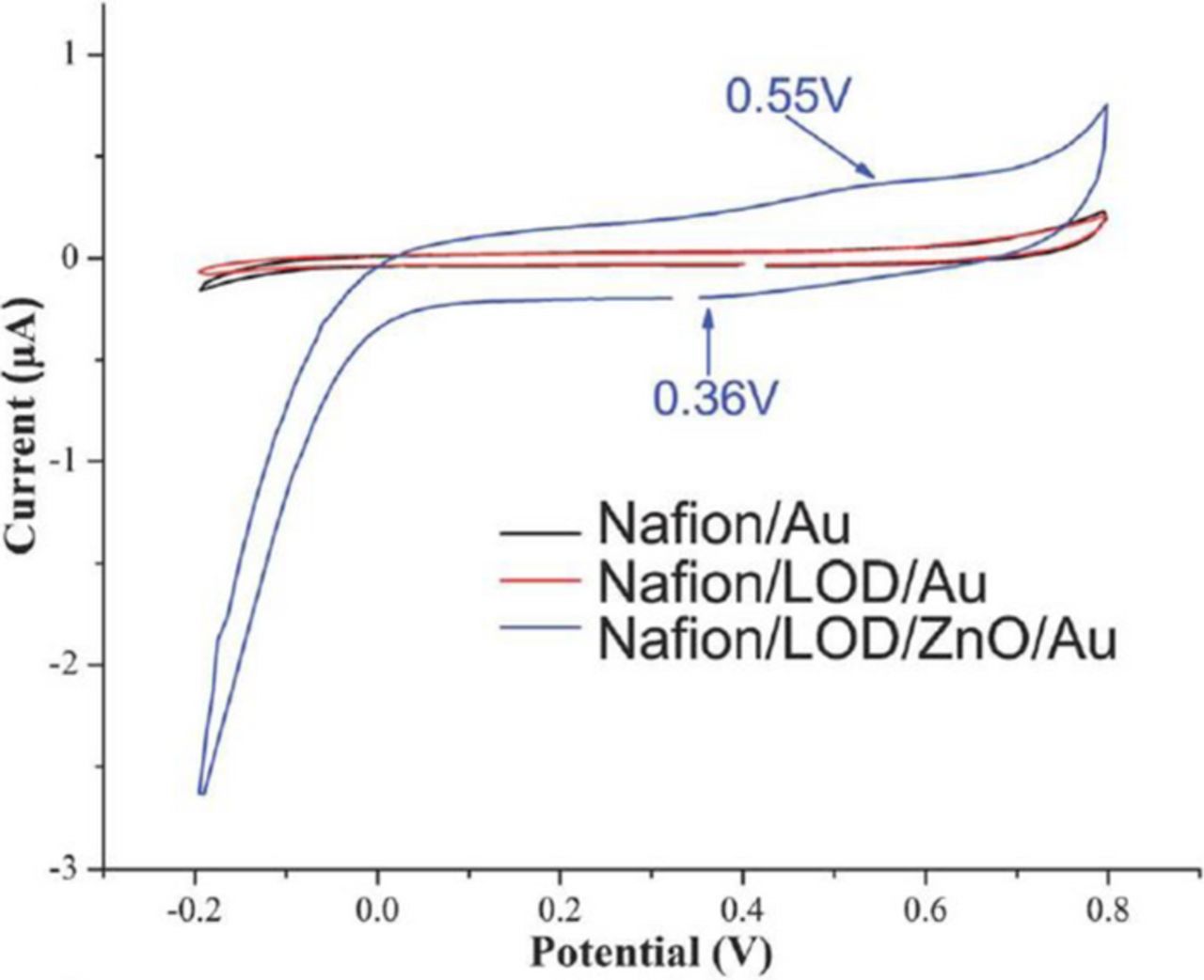
Lei et al.27 fabricated L-lactic acid sensors based on ZnO nanotetrapods which form a three-dimensional network that facilitates charge transfer providing numerous conductive paths from the LOD to the electrode. When tested in PBS, the Nafion/LOD/ZnO nanotetrapods/Au electrodes showed a sensitivity of 28.0 μA mM−1 cm−2 a detection limit of 1.2 μM, a low apparent Michaelis–Menten constant of 0.58 mM, and a good linear response in the range of 3.6 μM–0.6 mM. The effect of the ZnO nanotetrapods on sensing properties of the working electrode was studied by cyclic voltammetry. As compared to Nafion/Au (black curve) and Nafion/LOD/Au electrodes (red curve) fabricated without ZnO nanostructures, the Nafion/LOD/ZnO/Au electrode (blue curve) showed much better current response with two peaks of oxidation and reduction at 0.55 and 0.36 V, respectively (see Fig. 10).
Figure 10. CV curves of Nafion/Au electrode (black curve), Nafion/LOD/Au electrode (red curve), and Nafion/LOD/ZnO/Au electrode (blue curve) in PBS (pH 7.4) at a scan rate of 50 mV s−1 . Reproduced in part from Ref. 27 with permission of The Royal Society of Chemistry.
Comparative studies of lactic acid potentiometric biosensors based on ZnO nanorods and ZnO thin films revealed that the ZnO nanorods show better performance than ZnO thin films in terms of high sensitivity and low limit of detection.26,129 For L-lactic acid concentrations from 10−7 to 10−3 M, LOD/ZnO nanorod based electrodes demonstrated four times higher sensitivity (9.97 mV/decade) than that for LOD/ZnO film based devices (41.33 mV/decade). In agreement with earlier studies,126 the response of the biosensor was maximum for pH values from 6 to 9.
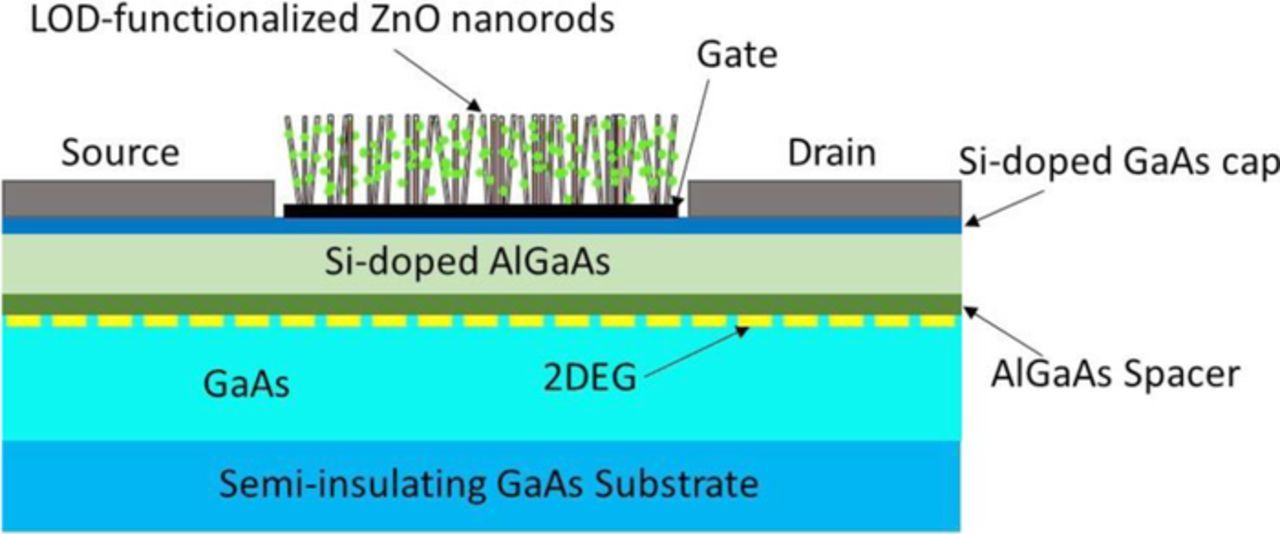
Heterojunction field effect transistors (HFETs) based on semiconductor heterostructures, such as GaN/AlGaN130 and GaAs/AlGaAs,131 have found application as platforms for L-lactic acid sensors. Figure 11 shows a schematic sketch of a ZnO-gated GaAs/AlGaAs HFET biosensor. A two-dimensional electron gas (2DEG) channel, forming at the AlGaAs/GaAs interface, with high electron sheet carrier concentration, is located near the surface at the heterostructure interface, so that a minor change in electrical charge at the gate surface results in the change in electron concentration in the 2DEG channel, which would then lead to a change in the source-drain current. For lactic acid detection, the gate area is functionalized with LOD attached to ZnO nanorods. The ZnO nanostructures on the gate surface increase the total sensing area and thus sensitivity could be significantly increased. The change of the current signal is amplified through the HFET, which allows detection of very low concentrations of lactic acid: detection limit as low as 3 pM was reported.131 In addition, because no reference electrode is required, the volume of the sample needed for measurements can be minimized, which makes HFET biosensors useful for measuring lactic acid levels in exhaled breath condensate as a surrogate for blood-based lactate measurements.132
Figure 11. Schematic sketch of ZnO nanowires-gated AlGaAs/GaAs HFET biosensor.
Uric Acid Biosensors Based on ZnO Nanostructures
Uric acid, a common component of urine, is the end product of purine metabolism in human body. The normal level of uric acid in human serum varies from 25 to 75 mg/100 mL.133 Increased uric acid concentrations in human blood (hyperuricemia) or urine (hyperuricosuria) can be a symptom of several diseases, including gout, Lesch–Nyan disease, cardiovascular disease, diabetes, kidney stones, leukemia, and pneumonia,134 while lower levels are associated with multiple sclerosis135 and Parkinson disease.136 The enzyme generally used to sensitize ZnO surface for uric acid sensing is uricase,24,25,137–143 (IEP ∼ 4.3 or 4.6) which can be immobilized on the ZnO surface. Sensing of uric acid (C5H4N4O3) is based on the following reaction catalyzed by uricase:144
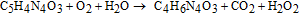
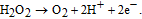
The products of the oxidation reaction are allantoin (C4H6N4O3), carbon dioxide, and hydrogen peroxide. In the presence of water, allantoin accepts a proton converting it to allantoinium ion, which in turn interacts with ZnO and produces potential change at the electrode.
Tzamtzis et al.139 fabricated uric acid sensors based on ZnO nanowires sensitized with an uricase-containing lipid film. Uricase was incorporated into the lipid film prior its polymerization on the ZnO nanowire surface. The use of the lipid film containing a positively charged lipid enhances the concentration of uric acid around the electrode, thus improving sensing performance. The potentiometric sensitivity of the lipid/ZnO sensor (61 mV/decade) was found to be twice as large as that of the sensor fabricated without a lipid film (32 mV/decade). The sensor also showed a low detection limit, fast response, good selectivity, reproducibility, thermal stability, linearity, and stability (see Table V).
Table V. Comparison of uric acid biosensors based on ZnO nanostructures.
| Electrode | Sensitivity | Linear range | Detection limit | Response time | Apparent Michaelis constant Kapp | Life time / % of original bioactivity | Ref. |
|---|---|---|---|---|---|---|---|
| Nafion/Uricase/ZnO nanorods/Ag electrodes | 239.67 μA cm−2 mM−1 | 0.01–4.56 mM | 5 nM | ∼3 s | 0.025 mM | 7 weeks of storage / ∼92% | 143 |
| Uricase/ZnO nanorods/GCE | 5.0 × 10−6 – 1.0 × 10−3 mol L−1 | 2.0 × 10−6 mol L−1 | 0.238 mM | 20 days / 80.5% | 24 | ||
| Nafion/uricase/ZnO nanoflakes/Al/Au-coated plastic substrates | ∼66 mV/decade | 500 nM–1.5 mM | 8 s | 3 weeks / 80% | 144 | ||
| PDDA/uricase/ZnO NPs/MWNTs/pyrolytic graphite | 393 mA cm−2 M−1 | 5 μM–1 mM | 2.0 mM | 160 days / 89% | 25 | ||
| Nafion/uricase/ZnO nanowires/Au-coated plastic substrates | 32 mV/decade | 1–1000 μM | 6.25 s | > 3 weeks / 80% | 137 | ||
| Nafion/uricase/ZnO nanotetrapods/Au | 80 μA mM−1 cm−2 | 0.8 μM–3.49 mM | 0.8 μM | 9 s | 72 h / 96% | 137 | |
| Uricase-containing lipid film/ZnO nanowires/Au-coated plastic substrates | 61 mV/decade | 1.0–1000 μM | 0.4 μM | 6 s | 3.89 | > 3 weeks/>90% | 139 |
| Nafion/uricase/ZnO wire/Au | 89.74 μA mM−1 cm−2 | 0.1–0.59 mM | 25.6 μM | 0.99 mM | 120 h / ∼86% | 145 | |
| Nafion/Uricase/ZnO nanosheets/Ag/Si | 129.81 μA mM−1 cm−2 | 0.05–2.0 mM | 0.019 μM | 5 s | 0.026 mM | 30 days / 97% | 140 |
| Uricase/single ZnO nanowire-based FET | 1 pM–0.5 mM | 1 pM | several ms | 141 | |||
| Nafion/uricase/ZnO nanotetrapods-modified AlGaAs/GaAs HFET | 0.2 nM–0.2 mM | 0.2 nM | ∼1 s | 142 | |||
| ZnO/CuxO/ polypyrrole/GCE | 0.5–70 μM | 0.2 μM | 153 | ||||
| ZnO/PANI/reduced graphene oxide | 100–1000 μM | 0.122 μM | 3 weeks / 90% | 151 | |||
| Au nanoparticles/ZnO nanorods/GCE | 10–400 μM | 2.375 μM | 146 | ||||
| ZnO nanowires/graphene foam/ITO/glass | 4.24 μA mM−1 | 0.1–60 μM | 1 nM | 147 | |||
| RGO–ZnO/GCE | 3–330 μM | 1.08 μM | 1 week / 97% | 148 | |||
| Carbon fibers/ZnO core–shell hybrids | 20–200 μM | 6.7 μM | 1 month / 97% | 149 | |||
| ZnO quantum dots/reduced graphene oxide sheets/MWCNTs/Ni foam/GCE | 70.537 μA mM−1 | up to 1.1 mM | 3.89 μA | 150 |
Zhao et al.145 designed an uric acid biosensor based on an individual ZnO nanowire. This work was motivated by a presumption that the performance of the biosensor based on disordered ZnO nanostructures may be deteriorated because of material and electrical discontinuity between the multi-nanowires, while the device based on an individual ZnO nanowire should exhibit outstanding electron transport properties resulting in the efficient electron transfer to the electrode. The amperometric response of the Nafion/uricase/ZnO wire/Au electrode was tested in the PBS (0.01 M, pH = 7.4). The designed sensor exhibited a sensitivity of 89.74 μA mM−1 cm−2 in the linear range of 0.1 to 0.59 mM, good selectivity, reversibility, and stability (see Table V). Unfortunately, the authors did not provide a comparative of this single-wire based sensor with those utilizing multiple nanowires, so the evidence on the superior performance of the single-wire design is inconclusive. As seen from Table V, multiple groups have reported better performance for sensors based on ZnO nanostructures.
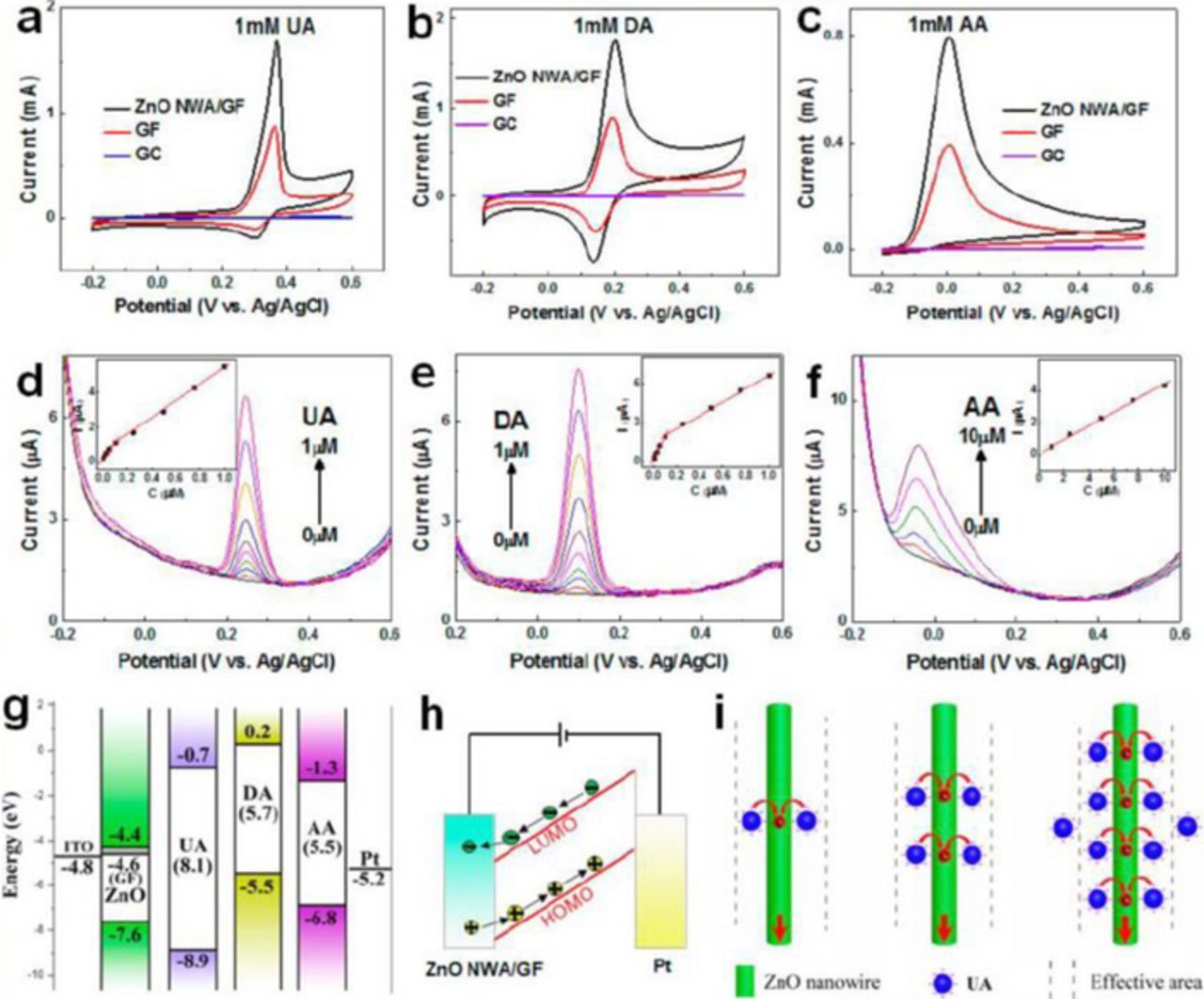
In recent years, non-enzymatic sensors based on various nanocomposites, such as ZnO nanorods-Au NPs,146 ZnO nanowires-graphene foam (GF),147 reduced graphene oxide-ZnO,148 carbon fibers-ZnO core–shell hybrids,149 reduced graphene oxide-intercalated ZnO quantum dot nanoballs,150 have been developed for simultaneous detection of uric acid, ascorbic acid, and dopamine. Since these species coexist in human serum and extracellular fluid of the central nervous system, there is a need for techniques for their sensing without cross interference. For example, Yue et al.147 used ZnO nanowires grown on 3D graphene foam to selectively detect uric acid, dopamine, or ascorbic acid by a differential pulse voltammetry (DPV). ZnO nanostructures exhibit high catalytic activity and also provide direct pathways for fast electron transport, while the porous structure of the graphene foam facilitate ion diffusion, offers large surface area and numerous active sites, and also possesses high electrical conductivity. As a result, the oxidation current density of the ZnO/GF/ITO electrode was considerably enhanced. Figures 12a–12c show cyclic voltammetry (CV) measurements for ZnO nanowire/graphene foam, graphene foam, and bare glassy carbon (GC) electrodes in 1 mM uric acid, dopamine, and ascorbic acid, respectively. One can see that, in all the cases, the ZnO/GF electrode exhibited the highest oxidation current, while the oxidation potentials for these species are different. DPV measurements with the ZnO/GF electrode at different concentrations of uric acid, dopamine, and ascorbic acid revealed that the current increases linearly with the analyte concentration (Figs. 12d–12f).
Figure 12. Electrochemical detection of uric acid (UA), dopamine (DA), and ascorbic acid (AA). (a)-(c) CV curves of the ZnO/GF, GF, and bare GC electrodes in 1 mM UA, DA, and AA, respectively, at a scan rate of 50 mV s−1. (d)-(f) DPV curves for UA, DA, and AA at different concentrations using a ZnO nanowires/GF electrode. Distinct oxidation potentials were observed at 0.25, 0.1, and 0.04 V for UA, DA, and AA, respectively. Insets: plots of the oxidation peak current vs concentration of each biomolecule. (g) Flat band model (LUMO and HOMO) of the ZnO nanowires/GF, UA, DA, and AA, and work function for the GF, ITO, and Pt electrodes. (h) Electron and hole transfer during oxidation of the biomolecules. (i) Schematic of the adsorbed biomolecule (UA) at different concentrations. The supporting electrolyte is a 0.1 M PBS solution (pH 7.4). DPV conditions: a pulse height of 50 mV, a step height of 4 mV, a pulse width of 0.2 s, a step time of 0.5 s, and a scan rate of 8 mV s−1. Reprinted with permission from Ref. 147. Copyright 2014 American Chemical Society.
The selectivity in the oxidation potential is attributed to the fact that the gaps between the lowest unoccupied (LUMO) and highest occupied molecular orbitals (HOMO) are different for different species. The oxidation potential is generally governed by the electron transfer from the analyte molecule to ZnO and hole transfer from the analyte molecule to the Pt electrode (Fig. 12h). From the flatband model shown in Fig. 12g, it is evident that the electron current depends on the height of the Schottky barrier forming at the ZnO/analyte interface between the ZnO conduction band and LUMO of the biomolecule, while the hole current is determined by the Pt work function and HOMO of the biomolecule,147,150 which results in different oxidation potentials for different species. This method was used to detect uric acid levels in human serum extracted from peripheral blood. The samples were taken from healthy individuals and patients with Parkinson's disease. For the measurements, the serum was diluted 500-fold using a 0.1 M PBS solution. It was found that the UA concentration was 25% lower for the patients with Parkinson's disease as compared to that for healthy individuals, proving that the proposed technique can be useful for diagnosing Parkinson's disease and monitoring disease progression.147
Nanocomposites involving conductive polymers were demonstrated to be useful for the multianalyte detection.151–153 Conductive polymers, such as polypyrrole (PPy) and polyaniline (PANI), allow the formation of selective electrode coatings distinguishing between species via hydrophobic and hydrophilic affinities, electrostatic interaction, or ion-exchange abilities.154 The conductive polymer-based nanocomposites provide a large surface area and high electrical conductivity, which enhances electron transfer in electrochemical reactions, and results in improved sensitivity, selectivity, and detection limits of biosensors. As an example, a nano ZnO nanosheets/CuxO nanowires/PPy modified electrode for simultaneous determination of uric acid, ascorbic acid, and dopamine was proposed by Ghanbari and Hajheidari.153 In their work, the procedure of the electrode fabrication was the following: PPy, CuxO nanowires, and ZnO nanosheets were deposited electrochemically from aqueous solutions on a glassy carbon electrode. PPy is a conducting polymer with a well-ordered chain structure and very high surface-to-volume ratio. The PPy nanofiber film provided large surface for attachment of the CuxO and ZnO nanostructures and transport of charge carriers, and lots of micropores between the nanofibers that could improve the incorporation of the analytes. Electrochemical measurements of uric acid, dopamine, and ascorbic acid in a 0.1 M Britton–Robinson (BR) buffer solution revealed well-separated voltammetric peaks of ascorbic acid, dopamine, and uric acid at about 0.26, 0.42, and 0.58 V, respectively. The detection limits for ascorbic acid, dopamine, and uric acid were found to be 25.0, 0.04, and 0.2 μM, respectively, which were very close to the values found for each analyte separately (25.0, 0.03, and 0.2 μM, respectively). The ZnO/CuxO/PPy-modified electrode showed also good reproducibility and selectivity against interfering species, including glucose, fructose, sucrose, lactose, cysteine, epinephrine, acetaminophen, serotonin, K+, Na+, Ca2+, Mg2+, Zn2+, NH4+ , Cl–, NO3–, SO42– , and HCO3. The proposed sensor was also successfully used for sensing uric acid in real urine samples. It should be mention, however, that the maximum of the peak currents of ascorbic acid, dopamine, and uric acid as well as the separation between peak potentials for AA-DA and DA-UA were observable at pH as low as 4.0, which is far from the natural pH of ∼7.4. For this reason, human urine samples were diluted 100 times with BR solution to obtain pH = 4.0.
PANI conducting polymer with porous structure was used to fabricate ZnO/polyaniline/reduced graphene oxide (RGO) nanocomposites for simultaneous determination of dopamine and uric acid.151 An electrode was fabricated by electrochemical deposition of flower-like ZnO nanostructures/polyaniline nanofiber/reduced graphene oxide nanocomposite (ZnO/PANI/RGO) on a glassy carbon electrode. The sensor showed no sensitivity to ascorbic acid, however it could delineate dopamine and uric acid oxidation peaks, and was found to be useful for determination of these species in the presence of a fixed concentration of ascorbic acid. Similar to the ZnO/CuxO/PPy-modified electrode, the ZnO/PANI/RGO-based sensor also demonstrated the best sensitivity and selectivity for low pH values (4.0) and was proved to be applicable for simultaneous determination of dopamine and uric acid in diluted samples of human urine and serum.
Metal Ion Biosensors Based on ZnO Nanostructures
Metal ions, such as Ca2+, Mg2+, K+, Na+, Fe3+, Zn2+, etc. play important roles in biological systems. Every metal ion has its own importance by acting as cofactors in enzymes, osmotic regulators, current carriers and consequently as factors in information processing and as integrator and stabilizers of proteins and lipids.155,156 Therefore, monitoring of metal-ion levels in biological fluids (blood, interstitial fluid, urine) is critical for human health. The general strategy to sensitize ZnO nanostructures for measurements of metal-ion concentrations involves conformal coating of electrodes with ion-selective (ionophore-containing) monolayer-thin membranes based on polyvinyl chloride (PVC). The main component of membrane-based ion selective electrodes for a particular type of the target ion is the ionophore (ion carrier), a species that selectively transfers a particular metal ion across the membrane. ETH 227,29 DB18C6,31,157 valinomycin,28 4,5-bis(benzoylthio)-1,3-dithiole-2-thione (Bz2dmit),32 12-crown-4,158 18 crown 6,159 and salicylaldehyde thiosemicarbazone (ST) ionophores were used for detection Na+, Ca+, K+, Mg2+, Zn2+, Fe3+, and Sr2+ ions, respectively.
Asif et al.30 fabricated a Ca2+ sensor by utilizing functionalized ZnO nanorods as an extended gate of a commercial metal oxide semiconductor field effect transistor (MOSFET). In extended-gate FETs (EGFETs), potential generated at the surface of the reference electrode is added to the gate voltage, and thus the source-drain current depends on the concentration of the ion of interest in the sample solution. The ZnO nanorods grown on a 0.25-mm-thick silver wire were covered with PVC membrane containing Ca2+-specific ionophore (DB18C6). The ZnO-nanorod electrode was externally connected at the gate terminal of a MOSFET in series as shown in Fig. 13a. The change in the interfacial potential on the ZnO electrode due to its interaction with Ca2+ ions was converted into the change in the drain current of the MOSFET, and in the Ca2+ signal was amplified. The current response of the fabricated sensor studied in an aqueous solutions of CaCl2 was found to be linear in the Ca2+concentration range of 1 μM to 1 mM (Fig. 13b).
Figure 13. (a) Experimental setup for the Ca2+ detection. (b) Change of the drain current as a function of Ca2+ ion concentration in range from 1 μM to 1 mM. Reprinted from Ref. 30 with permission from Elsevier.
For intracellular measurements of Ca2+ concentration, the same team73,157 fabricated potentiometric sensors based on ZnO nanorods. The nanorods were grown by the aqueous solution method on Ag-coated borosilicate-glass capillaries (0.7 μm in diameter) and functionalized with PVC membrane containing DB18C6. The experimental setup was the same as that used for intracellular monitoring of glucose, as shown in Fig. 3d. The ZnO-modified working electrode showed a Ca2+-concentration-dependent electrochemical potential difference with respect to an Ag/AgCl reference microelectrode. First, the sensor was calibrated with a buffer solution of CaCl2 (pH ∼ 7), and the potential difference was found to be linear over a large concentration range of 100 nM to 10 mM. Then, the Ca2+ concentration was measured in human fat cells and in frog egg cells. The intracellular Ca2+ concentrations in the human and frog cells were found to be 123 ± 23 nM and 250 ± 50 nM, respectively, which are in good agreement with published data.160,161 SEM studies of the functionalized working electrode after intracellular measurements revealed no evidence of dissolution of the ZnO nanorods, which was attributed to the fact that the ZnO surface was protected with the polymeric membrane.73 The same approach was used for sensing K+,28,73,162 Na+,29,73 and Mg2+32,73 in the context of intracellular measurements. As in the case of Ca2+, so fabricated ion sensors exhibited a decent sensitivity, stability, and selectivity in the presence of interfering ions. The intracellular ion concentrations measured in human adipocytes and frog oocytes were consistent with values found in the literature. The performances of the sensors are listed in Table VI.
Table VI. Comparison of metal ion sensors based on ZnO nanostructures.
| Ion | Electrode | Sensitivity | Linear range | Detection limit | Response time | Ref. |
|---|---|---|---|---|---|---|
| Ca2+ | DB18C6/ZnO nanorods/Ag wire EGFET | 113.92 mV/decade | 1 μM–1 mM | 20 s | 30 | |
| Ca2+ | DB18C6/ZnO nanorods/ Ag-coated glass capillary | 29.67 mV/decade | 100 nM–10 mM | 31 | ||
| Ca2+ | DB18C6/ZnO nanorods/Ag wire | 26.55 mV/decade | 0.1 μM–0.1 M | < 1 min | 157 | |
| K+ | valinomycin /ZnO nanorods/ Ag-coated glass capillary | 41.5 mV/decade | 25 μM –125 mM | 1 μM | < 30 s | 28 |
| Na+ | ETH 227/ZnO nanorods/ Ag-coated glass capillary | 72 mV/decade | 0.5 mM–100 mM | 29 | ||
| Mg2+ | Bz2dmit/ZnO nanorods/ Ag-coated glass capillary | 26.1 mV/decade | 500 nM–100 mM | 32 | ||
| Zn2+ | 12-crown-4/ZnO nanorods/Au-coated glassy electrode | ∼35 mV/decade | 1 μM –100 mM | < 5 s | 158 | |
| Sr2+ | ST/ZnO nanorods/Au-coated glassy electrode | 28.65 mV/decade | 1 × 10−6–5 × 10−2 M | 10 s | 129 | |
| Fe3+ | 18 crown 6/ZnO nanorods/Ag wire | 70.12 mV/decade | 10−6–10−2 M | 159 |
Ibupoto et al.158 demonstrated selective Zn2+ ion electrode based on ZnO nanorods grown on gold-coated glassy electrodes by low temperature, low cost hydrothermal method. The ZnO nanorods were functionalized with zinc-ion selective membrane (12-crown-4) in conjunction PVC. The potentiometric Zn2+ ion sensors based on the functionalized ZnO electrode in combination with an Ag/AgCl reference electrode was tested in PBS (pH = 7.4). The proposed device showed a good linearity in the concentration range from 1 μM to 100 mM, a sensitivity of 35 mV/decade, and a fast response time of less than 5 s. The sensors exhibited good repeatability, reproducibility, and negligible response to interfering ions, including Ca2+, Mg2+, K+, Fe3+, and Cu2+. The effect of pH on the electrochemical response was studied in a 5-mM Zn(NO3)2 solution in the pH range of 4 to 10. The signal reached its maximum at pH ∼ 7.4, which corresponds to pH of biological fluids, while at lower pH values it decreased because of dissolution of the membrane in an acidic medium, and at higher pH values the signal decreased due to dissolution of ZnO. Moreover, it was demonstrated that the proposed sensor can be used in the potentiometric titration of Zn2+with ethylenediamine tetra acetic acid (EDTA).
pH Sensors Based on ZnO Nanostructures
The monitoring of pH value is important in many fields, including clinical, pharmaceutical, food, environmental, and industrial applications. Due to the high surface-to-volume ratio, nontoxicity, biocompatibility, and high electrochemical activity, and unique amphoteric properties (reacting with both acidic and alkaline solutions) ZnO nanostructures have been proposed as pH-sensitive membranes. The use of ZnO nanostructures as a pH sensor is based on the activity at the electrolyte/ZnO interface, since ZnO surface have high density of specific adsorption sites for H+ and OH− ions. These sites may act as proton donors or acceptors depending on the solution pH, leading to electrochemical potential response of ZnO nanostructures to variations in pH.The response of the sensor depends on the density of these site as well as on the reaction rate constants of protonation/deprotonation of these sites. Quantitativeestimations13,163 of ZnO nanorod pH response performed within the framework of site-binding theory164 has predicted the maximum Nernstian sensitivity of 59.1 mV/pH at 25°C in the wide range of pH values from 1 to 14.
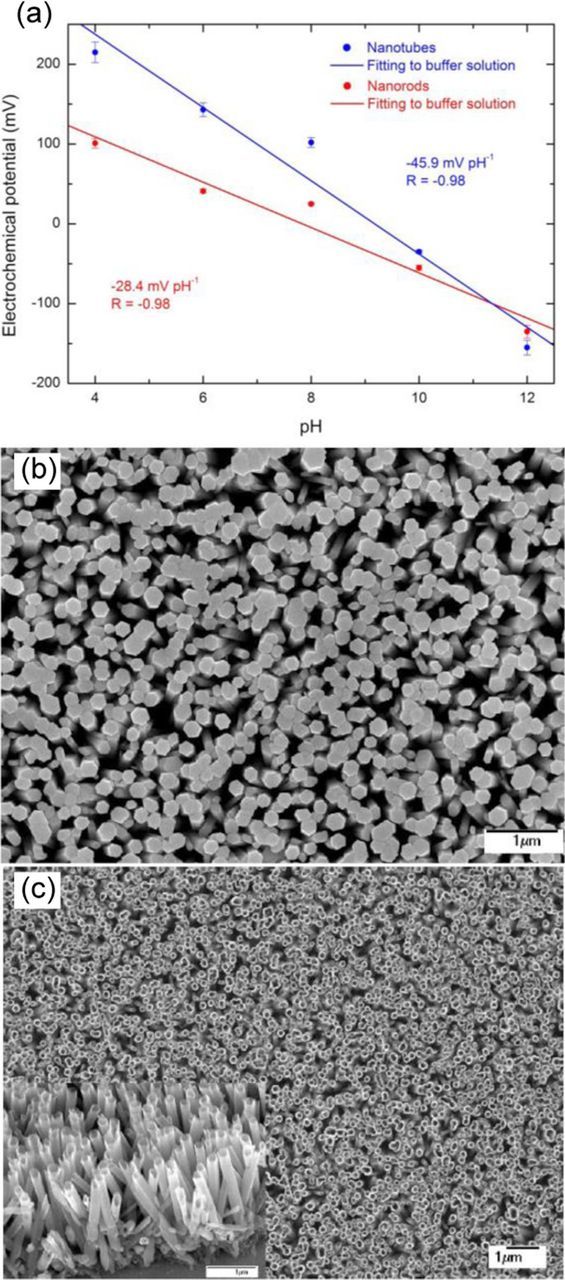
In 2005, Kang et al.165 reported on the electrical response of a single ZnO nanorod to variations of the pH in electrolyte solutions. A change in surface potential at the ZnO/liquid interface was readily measured as a linear change in conductance of the single nanorod in the range of pH from 2 and 12 of 8.5 nS/ pH with a resolution of ∼0.1 pH. In succeeding years, the pH sensors based on ZnO nanorods,13,166–169, nanotubes,13 nanowalls,170 nanowires,171 and nanoflakes171 have been developed. As expected,163,168 the surface morphology (i.e. surface-site density) was found to have pronounced effect on the pH sensing performance. Zhang et al.171 reported that the nanowire structures having higher surface-to-volume ratio than the nanowire–nanoflakes hybrids showed higher sensitivity (36.65 mV/pH for nanowires vs 34.74 mV/pH for nanowires–nanoflakes). Fulati et al.13 have found than ZnO nanotubes provide sensitivity as high as twice that of ZnO nanorods due to higher surface-to-volume ratio as well as higher level of surface and subsurface oxygen vacancies. As shown in Figure 14, the electrochemical potentials of both nanotube- and nanorod-based electrodes show linear dependence on the pH value in the range from 4 to 12, but the sensitivity of ZnO nanotube electrode is much higher: about 45.9 mV/pH as compared to that of the same dimension ZnO nanorod electrode (28.4 mV/pH). Using very thin (20 nm) ZnO nanowalls, Maiolo et al.170 have fabricated a fully flexible pH sensor based on extended gate polysilicon thin-film transistor (TFT). The device showed a near-ideal Nernstian response of 59 mV/pH. Such an improvement was related to the very large surface-to-volume ratio of the nanowalls as well as to the presence of surface defects and/or vacancies acting as bonding sites for protonation/deprotonation reactions.
Figure 14. (a) Experimental measurements of electrochemical potential vs pH for ZnO nanorods and nanotubes immersed in a buffer solution. SEM images of ZnO (b) nanorods and (c) nanotubes (insert shows tilted cross sectional view of nanotubes). From Ref. 13.
In addition to the surface morphology, chemical functionalization of ZnO nanostructures can enhance the sensor stability and extend its pH sensing range.166,171 Kumar et al.166 reported that bare ZnO nanorods were etched in an acidic environment (pH = 4.3), which made impossible pH sensing. To overcome this problem, the nanorods were functionalized with 3-aminopropyltriethoxysilane (APTES), which not only protected ZnO from etching in both acid and alkaline solutions, but also improved pH sensitivity, allowing pH measuring in the pH range from 4.3 to 9.2 with a sensitivity of 50.1 mV/pH. For APTES-modified ZnO nanostructures, Zhang et al.171 were able to increase device sensitivity and get a pH response at 2, which was not possible for bare ZnO (see Table VII). It has been also demonstrated that doping with Al can improve the pH sensitivity of ZnO nanostructures.83,172 Wang et al.172 have revealed that the pH sensing properties of EGFET sensors based on hydrothermal Al-doped ZnO (AZO) nanostructures depend on the Al content. The optimum Al concentration was found to be 3 at.%, which provided high sensitivity of 57.95 mV/pH (as compared to 35 mV/pH for undoped ZnO) in a wide range of pH from 1 to 13.
Table VII. Comparison of pH sensors based on ZnO nanostructures.
| Electrode | Sensitivity, mV/pH | Linear pH range | Response time | Ref. |
|---|---|---|---|---|
| ZnO nanowalls based EGTFT | ∼59 | 1–9 | 170 | |
| APTES-modified ZnO nanorods/SiO2/Si substrates | 50.1 | 4.3–9.2 | 166 | |
| ZnO nanowires-based EGFET | 36.65 | 4–9 | 171 | |
| ZnO nanowires-nanoflakes based EGFET | 34.74 | 4–9 | 171 | |
| APTES-modified ZnO nanowires-based EGFET | 43.22 | 2–9 | 171 | |
| APTES-modified ZnO nanowires-nanoflakes based EGFET | 41.58 | 2–9 | 171 | |
| AZO nanostructures-based EGFET | 57.95 | 1–13 | 172 | |
| ZnO nanorods/borosilicate glass capillary | 51.881 | 4–11 | 167 | |
| ZnO nanorods /Au/Cr/glass | 28.4 | 4–12 | <100 s | 13 |
| ZnO nanotubes /Au/Cr/glass | 45.9 | 4–12 | <100 s | 13 |
| ZnO nanorods-based EGFET | 15.4 | 4–12 | 169 |
Al-Hilli et al.167 used ZnO nanorods as an intracellular working electrode for in vivo measurements of pH in a human cell. For this purpose, ZnO nanorods were grown on the tip of a silver-coated borosilicate glass capillary (0.7 μm in diameter). The nanorods were ∼700 nm long with 80 nm in diameter, so the total diameter of the tip was 1.5 μm. The ZnO nanorods functionalized with H3O+ and OH− groups showed an electrochemical potential difference against theAg/AgCl intracellular reference microelectrode. To calibrate the sensor, the electrochemical response to the changes in buffer electrolyte pH was measured for a pH range of 4–11. A linear dependence on pH value was revealed, with a sensitivity of 51.881 mV/pH at 22°C. Then, the sensor was used for measurement of intracellular pH in a single human adipocyte or fat cell. Figure 15 shows an optical image and a schematic diagram for the intracellular potentiometric measurements. The working and reference electrodes were gently inserted into the cell via hydraulic fine adjustments past the cell membrane and elongating a short way into the biological cell. The signal reading was recorded once the ZnO nanorods based electrode was inside the cell. The measured pH value of 6.81 was close to the reported values for intracellular pH in rat brown adipocytes (6.95–7.57)173 or in rat hepatocytes (6.85–7.05)174 obtained by using indirect determination of pH. It should be mentioned that the introduction of the electrodes into a single cell's cytoplasm to measure the intracellular pH does not visibly seem to affect cellular viability. This study demonstrated that the ZnO nanorod electrode holds promise for minimally invasive monitoring of pH values inside living cells. Future applications of the ZnO nanorod pH electrode could include reducing the size of the sensing tip and achieving additional multianalyte detection measurements.
Figure 15. Optical image and schematic diagram illustrating intracellular pH measurements performed in a single human fat cell using ZnO nanorods as a working electrode with a Ag/AgCl reference microelectrode. Reprinted from Ref. 167, with the permission of AIP Publishing.
Outlook
The unique combination of physical and chemical properties of nanostructured ZnO has made it an excellent platform for chemical sensing of various analytes via the immobilization of proteins, including enzymes and antibodies, or chemical groups. The interest in ZnO-based biosensors is further fueled by fast electron transport exhibited by ZnO nanostructures, and improved catalytic properties which paves a new avenue for novel functionalities in a wide range of biomedical applications. The aforementioned features enabled impressive progress in the development of ZnO-based sensor devices toward their biomedical applications. Electrochemical sensors based on ZnO nanostructures have been realized and applied for detection of a variety of metal ions and physiologically important metabolites such as glucose, cholesterol, L-lactate, uric acid, and others. Impressively low detection limits (down to the nM range), relatively wide linear response ranges, and high sensitivity and selectivity have been demonstrated for a variety of analytes. Remarkably high sensitivity and selectivity of biosensors based on ZnO nanostructures provide the possibility of developing analytical configurations for disease markers, biological and infectious species, and life threatening agents toward early diagnostics. ZnO biosensors show promise for the multi-analyte quantification using proper biosensing schemes. Also noteworthy is that high sensitivity of ZnO-nanorod-based devices has led to the demonstration of selective electrochemical sensors for intracellular measurements of glucose, pH, and metal ions, which enable minimally invasive dynamic analysis of single cells.
Further developments in ZnO-based biosensors require a multidisciplinary approach which would address different aspects in the realm of controllable methods for nanostructure growth as well as indepth understanding of surface and interface chemistry and charge transport. Despite recent successes, there remain several challenges which must be properly addressed for transitioning ZnO-based miniature biosensors from laboratory to adaptation in healtcare. Among the most critical issues are (1) cytotoxicity of ZnO, particularly in the nanostructured form, which is not well studied and insufficiently understood and (2) dynamic chemical stability of ZnO nanostructures during repeated use should be criticaly examined. The latter is of no consequence for one time applications, but may become a problem in continuous monitoring of human health.