Abstract
The electrolytic decomposition of metal oxides to metal and oxygen is an extractive metallurgy principle that, when coupled with carbon-free electricity, drastically mitigates the global warming impact of metal production. The present perspective discusses the electrochemical engineering features of an unconventional electrolyte, molten oxides. A survey of its thermodynamic properties suggests exceptional features, both in terms of applicability to multiple metals and operation at high temperature to produce liquid metal. The review of molten oxides' transport properties indicates that an unprecedented throughput can be envisioned, a promising feature for tonnage production. However, our ability to define the optimal electrolyte composition with regard to energy consumption is rendered limited due to the lack of predictive tools for both of the reviewed properties. A look at the state of the art in electrode materials reveals that quantitative design criteria remain to be developed for both the cathode and the anode. Finally, the applications of electrochemistry in molten oxides are reviewed; thereby confirming most of the anticipated theoretical features.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
Metals have been essential materials for mankind for more than 2 millennia, and they remain the most important materials in terms of market value. Because of their centrality in the structural framework of the modern world, some metals have developed a commodity status, much like water or food. In the last two decades, an unprecedented increase in the global demand for metals has occurred (Figure 1a). This trend is expected to continue with the world population predicted to reach 9 billion by 2020.
Figure 1. a. Global annual production of iron and aluminum (x10) since 1950 in millions of metric tonne per annum [data from WorldSteel and WorldAluminium]. b. Global warming and acidification potentials per metric tonne of primary metal (data from Ref. 1).
Such growth does not come without challenges, particularly when it comes to the sustainability of primary metal production. Current extraction and processing routes are indeed characterized by large capital and environmental impacts. The former issue leads to difficulty in financing new facilities, particularly in developing countries that are poised to become significant consumers of primary metals. The existing technical paradigm necessitates multi-billion dollar investments that in turn require exceptional profits and short-term metal price stability in order to be supported by financing organizations. The environmental impact, illustrated in Figure 1b via the specific global warming and acidification potentials of key metals, can hinder the implementation of greenfield plants in countries with the most stringent environmental regulations. Additionally these emission potentials constitute a threat to the market in the eventuality of their taxation.
The present perspective offers first to reconsider the existing paradigm for the extraction of metals from oxides, which accounts for the majority of metal resources in tonnage and value. In particular, this document focuses on the use of electricity for the direct decomposition of metal oxides. The first section discusses the metallurgical strategies to extract a metal (M) from its oxide (MO) and the characteristics of the direct electrolytic decomposition pathway. In the second section, an argument is made for the use of molten oxides as a possible supporting electrolyte by reviewing their thermodynamic and transport properties. Possible electrode materials are discussed as well. Finally, the third section reviews the technical advancements reported to date using such an electrolyte and highlights its application for iron, manganese, silicon and oxygen production.
Fundamentals of Metal Extraction: The Electrolytic Path
The coupling approach
Presently, industry faces the need to process oxide feeds of various grades and compositions. It must do so carefully in order to ultimately produce metal with stringent specifications at minimal cost. Both of the above requirements offer engineering challenges entirely dictated by the chemical basis of the oxide reduction reaction, i.e. the decomposition step. The main energy consumption for primary metal production is precisely at this extractive metallurgy step,2 and it is therefore proposed to review its fundamental objectives. To simplify the argument, a chemically pure divalent metal oxidea feed is considered. The main target for extractive metallurgy is then to accomplish the decomposition reaction 1:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/162/1/E13/revision1/jes_162_1_E13eqn1.jpg)
The inverse of reaction 1 occurred during earth's early history, and is responsible for the formation of the rocks and ores that now compose its surface. Metal extraction aims at reversing this geological event and obtain the metal in either the solid or liquid state. The thermodynamic characteristics of reaction 1 are presented in Figure 2a. It highlights that the decomposition is non-spontaneous and requires mainly work (Gibbs free energy, dG) at low temperature, and heat (difference between Enthalpy, dH, and Gibbs free energy) at high temperature.3 In order for reaction 1 to occur toward the right, a 'coupling' reaction 2 involving a reductant is typically employed. Reaction 2 is currently used industrially for iron production:4
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/162/1/E13/revision1/jes_162_1_E13eqn2.jpg)
Figure 2. a. Gibbs free energy (plain lines) and heat (dotted lines) required for the isothermal and isobaric (atmospheric pressure) decomposition of respectively nickel and iron oxide (reaction 1) to metal; and carbon dioxide to carbon (opposite of reaction 2). b. Possible paths for iron extraction and some of their corresponding technologies for steelmaking (scheme reproduced from Birat5).
As seen in Figure 2a, reaction 2 provides sufficient dG to compensate the endergonicity of the metal oxide decomposition 1 at a moderate temperature between 200 and 1000°C. However, reaction 2 fails to provide the heat needed to maintain the temperature of the reacting system, because the entropy change for the complete combustion of carbon by oxygen to form CO2 is negligible. This fact practically leads to the use of a larger amount of carbon than stoichiometrically required according to reactions 1 and 2, only to maintain the heat balance of the process by pure combustion. The 'coupling' of 1 and 2 has been engineered at an exceptional level of refinement, for example for iron production, which energy and reductant consumptions are presently close to optimal.5
Independently of the amount of energy consumed, using carbon as a reductant clearly binds the metal industry to the generation of CO2 as a by-product, and supports the figures reported in Figure 1b. New paradigms are therefore considered, as summarized in Figure 2b. The substitution of carbon with hydrogen-rich fuels, e.g. syngas (CO+H2) or methane (CH4) allows for a reduction in the emissions thanks to the lower carbon content of the fuel. It however does not offer an actual decoupling between the energy requirements to run reaction 1 and CO2 emissions. Hydrogen oxidation also involves a coupling reaction and calls for the indirect and intensive use of electricity for its production. Clearly, the direct use of electrons is the only path to decouple energy requirements for metal production from reductants or by-products generation. It is the feasibility of this direct electrolytic approach that the current document first seeks to explore.
The direct decomposition approach
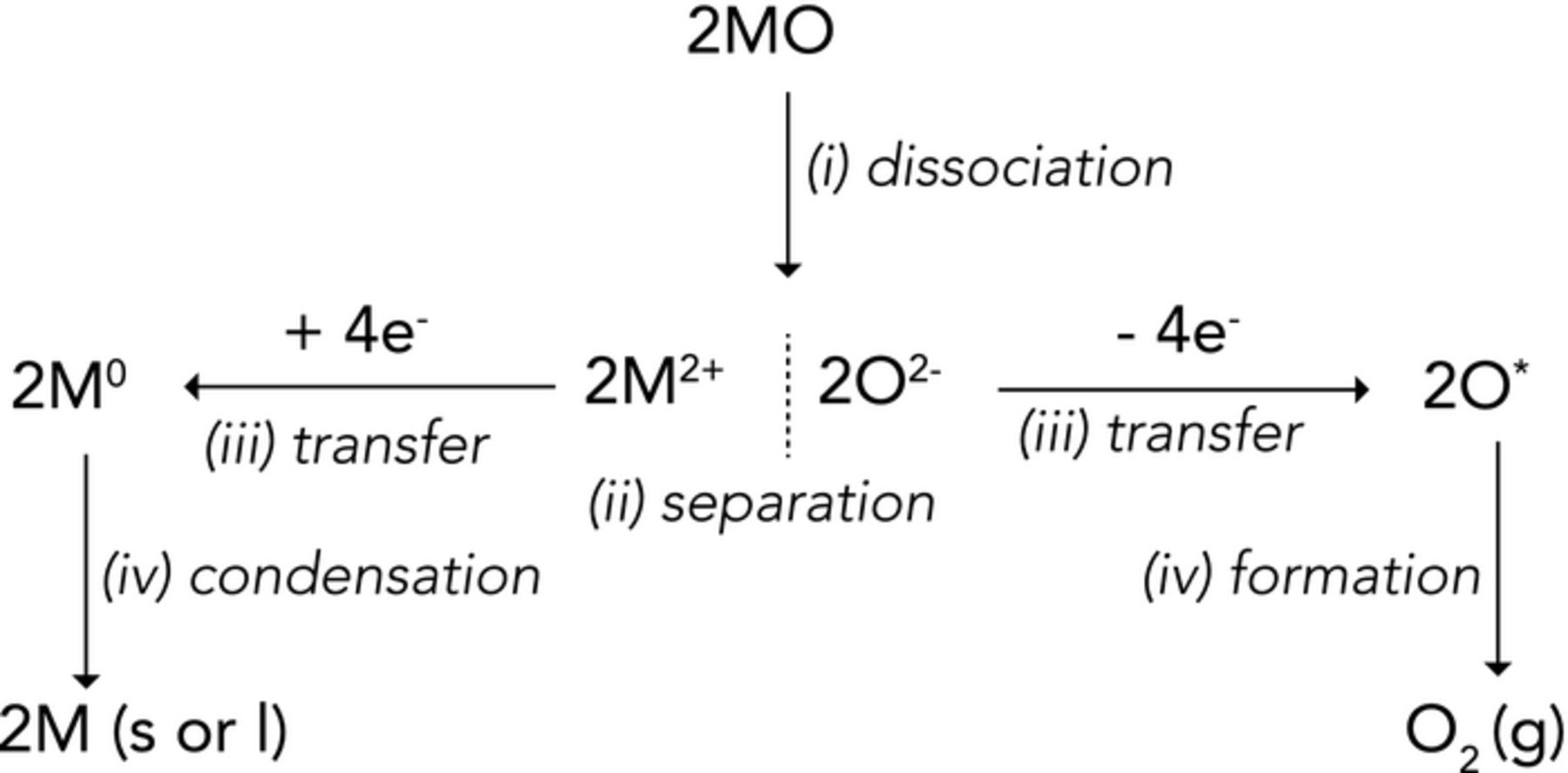
Under further consideration, reaction 1 can be envisioned as a succession of elementary steps, similar to a Born-Haber cycle. One such succession is illustrated in Figure 3. The corresponding steps are i) the dissociation of the oxide into its constitutive anions and cations - i.e. taking advantage of its electrolyte nature; ii) the separation of the charged species; iii) the transfer of electrons to the cations simultaneously with their removal from the anions to form the neutral atoms of each species; iv) the independent condensation of the metal and formation of the oxygen molecule. Such scheme shows that a supporting medium - to dissociate the oxide - as well as a separating device are likely to be required to perform this succession of physical events at a significant rate. In addition, steps iii) and iv) call for a simultaneous, localized, and controlled electron transfer. In principle, the energy required for those steps could be of various nature (e.g. a gradient of oxygen partial pressure or an extremely high temperature, see Ref. 3). Practically, electrolysis shows promise for such a purpose; following its description from Faraday:
Figure 3. Conceptual steps for the direct decomposition of a metal oxide to metal and oxygen gas.
'the use of electricity [...] would show some new conditions of action, evolve new views of the internal arrangements and changes of the substances under decomposition, and perhaps give efficient powers over matter as yet undecomposed'[6, (§451)] and 'the effect [of electrochemical decomposition] is produced by an internal corpuscular action, exerted according to the direction of the electric current and that is due to a force either superadded to, or giving direction to the ordinary chemical affinity of the bodies present.' [6, §518].
Direct electrolytic decomposition of oxides, provided it is conducted without consumption of carbon-containing materials (electrodes or container), eliminates direct (process) GHG emissions for metal extraction. In order to discuss the indirect GHG emissions associated with the generation of the required electricity, it is helpful to estimate the energy consumption for such an electrolytic process. Thermodynamics dictates that the decomposition of iron oxide (α-Fe2O3(s), hematite, room temperature) to oxygen and liquid metal (Fe) at 1600°C requires a minimum of 2600 kWh·tFe−1 (enthalpy balance). Additional energy will practically be needed to compensate for faradaic losses (e.g. 90% of selectivity, like for aluminum electrolysis) and heat losses (60% efficiency, typical for an electric furnace at such temperature). The electricity consumption for a practical, optimized process is therefore likely to be of the order of 3700 kWh.tFe−1. Figure 4 shows that if this amount of electricity is supplied by renewables or even by natural gas combustion, a mitigation of GHG emissions relative to those generated by existing primary steel production technologies can be accomplished. The recent and growing push toward hydro, solar, and wind energy harvesting to achieve de-carbonization of electricity production offers an even greater incentive to move toward direct electrolytic production of primary metals.
Figure 4. The indirect GHG emissions of electrolytic metal production can be estimated thanks to the specific electrical energy requirement, e.g. 3.7MWh.tFe−1 for the production of liquid iron and oxygen at 1600°C. Various electric power generation modes can be envisioned, which GHG impacts are reported elsewhere in tCO2.MWh−1.7 The corresponding GHG for electrolysis is compared with the existing state-of-the art integrated steel-plant emissions at 1.85tCO2.tFe−1. A negative percentage indicates that the electrolysis process would overall lead to a decrease in GHG emissions. Interestingly, and beyond the obvious mitigation obtained with C-lean electricity, the powering of an electrolysis plant with a natural gas fired power plant could lead to a reduction in GHG emissions from iron production.
The electrolytic challenge
The electrolytic challenge requires the discovery of a supporting medium or electrolyte that:
- acts as a solvent for the metal oxide feedstock,
- exhibits ionic conduction and a high rate of mass transport,
- is stable with respect to the gradient of chemical potential corresponding to the metal oxide decomposition.
Unfortunately solid metal oxide feedstocks rarely possess all such properties for practical realization. The supporting electrolyte is at the core of the separating device, physically isolating the products of reaction 1. Macroscopically, the separation and the electron-transfer steps occur via the application of a potential difference across the electrolyte thanks to and at the electrodes. The cathode (the electrode held at a lower potential), is where the electropositive metal ion is reduced to metal following reaction 1a:
![Equation ([1a])](https://content.cld.iop.org/journals/1945-7111/162/1/E13/revision1/jes_162_1_E13eqn3.jpg)
The anode (the electrode held at a higher potential) completes the electrical circuit by collecting the electrons via the oxygen evolution reaction 1b:
![Equation ([1b])](https://content.cld.iop.org/journals/1945-7111/162/1/E13/revision1/jes_162_1_E13eqn4.jpg)
The supporting electrolyte chemistry will therefore guide the choice of the two electrode materials to perform these reactions.
In the last twenty years, new physical arrangements have been devised using electrolytes with favorable physical chemistry properties, specifically those related to transport. Two examples of direct solid oxide reduction in contact with a cathode can be cited. At low temperature (100°C), the direct reduction of a suspension of solid iron oxide particles in an aqueous alkaline electrolyte8 has reached reasonable success at the pilot-scale. This success was in part due to previous studies of the topochemical electroreduction mechanism of the oxide particles at the cathode.9 At higher temperature, the remarkable stability of chloride electrolytes enables the reduction of metal oxide in direct contact with the cathode10–12 for the production of reactive and refractory metals. Another physical separation method has been put forward for the reduction of magnesium oxide to magnesium vapor in fluoride melts. It operates at a temperature between 1100°C and 1300°C using a zirconia-based membrane as a key separating component.13
One understudied medium is molten oxides. The technical developments reported to date describe the following principle for metal extraction: a mixture of oxides is a molten host for the dissolution of a metal oxide feedstock and acts as a supporting electrolyte for electrolysis. The corresponding process then matches the cycle described in Figure 3 at a temperature of operation high enough to produce liquid metal at the cathode. A thermodynamic advantage is inherent to the use of electricity at such high temperature: the main source of irreversibility - the Joule effect - can be exploited to maintain the process temperature. This feature is particularly beneficial for molten salts or oxides processes, which can then be conducted in "self-heated" conditions. This principle allows forming a frozen sidewall of electrolyte that acts as a container, a method currently applied for aluminum electrolysis cells. Such an approach proves to be particularly cost-beneficial because it alleviates the need for expensive container or lining materials.
The key component of an electrochemical process is therefore the supporting electrolyte, which in turn determines the electrode materials challenges to be addressed for successful operation. In the forthcoming, it is first proposed to discuss the electrolytic properties of molten oxides, and then to present the corresponding electrode materials challenge.
A Supporting Electrolyte and Two Electrodes
The history of electrochemical engineering shows that a satisfactory supporting electrolyte has to be designed prior to selecting the electrode materials. This section therefore reviews first the properties of molten oxides, considering the metal ion feedstock as a solute in binaries formed with silica. Such limitation to silicates is inherited from the prior art in molten oxide electrolysis, which has for the most part been conducted with silica-rich melts. The choice of binary systems is purely didactic, allowing illustration of common trends in properties with the fewest number of figures.
A high temperature solvent?
Metal oxide solubility at high temperature
Thermodynamic data for silica (SiO2)-containing oxide mixtures are plentiful due to their ubiquitous usage in industry. Silica is the most abundant constituent of the earth's crust and thus correspondingly inexpensive. The data presented here have been extracted from a commercial database (FactSage), but compilations are readily available in the open literature.14
Molten oxides mixtures with silica exhibit a high melting point and an exceptional range of thermal stability. Figure 5 highlights these features for a few relevant metal oxide binaries. These oxides are ranked according to their respective weight fraction (ratio of dark to overall area) at the lowest eutectic of their binary mixture in silica. Less than 40wt% (resp. 47 mol%) of those oxides is sufficient to form a melt at a temperature below the melting point of silica (1713°C for β-crystobalite). The maximum temperature of operation for most systems is high, limited by the formation of silicon monoxide gaseous species. Exceptions are the alkaline oxide containing systems, whose operating temperature is limited by their thermal decomposition, e.g. at around 1500°C for Na2O; and manganese containing systems which also exhibit a high vapour pressure at low temperature due to the half d-shell electronic structure of manganese. Thermal stability is an asset for the production of high-melting point 3-d transition metals in the liquid state. High temperature also allows fast reaction kinetics and semi-continuous operation thanks to periodic casting of the liquid metal product.
Figure 5. Range of thermal stability of some key SiO2-MyOz binaries at atmospheric pressure. The ratio between the darker and the overall area for each bar indicates the weight fraction of MyOz in the binary. The minimum temperature corresponds to the eutectic except for the 3-d transition metal oxides, for which the lowest temperature is equal or greater to the target metal melting point. For all compounds, the high temperature limit is arbitrarily fixed at a partial pressure of 10−3 atm in any of the constituents (SiO for all oxides except Na for Na2O, and the metal vapor pressure for the 3d-transition metals).
Oxide melts, because of their chemical nature, have the ability to dissolve a significant amount of 3-d metal oxide. Figure 5 shows the composition for three monoxides (manganese, nickel and iron monoxide as metal feed) at the lowest liquidus temperature achievable in their binary with silica. Concentrations higher than 40 wt% could be foreseen. In practice, the concentration in metal oxide feedstock is reduced for electrochemical engineering reasons (see Historical developments section), but compositions up to 25 wt% are not unrealistic. The corresponding metal ions concentration is higher than 10,000 mol·m−3, an exceptional number in comparison with typical industrial electrolytes (1,000 mol·m−3 and 7,000 mol·m−3, respectively for zinc and aluminum electrowinning).
From an extractive metallurgy standpoint, oxide melts based on silica exhibit a remarkable chemical stability. The thermodynamic minimum cell voltage required for the decomposition of the constitutive oxides (the 'electrochemical window' of operation, U°)b, is obtained from the Gibbs free energy change (ΔG°(1)) and the number of electrons transferred (n) in the reaction:
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/162/1/E13/revision1/jes_162_1_E13eqn5.jpg)
This calculation, illustrated in Figure 6, explains the successful application of molten oxides for the extraction of relatively noble elements such as nickel, iron, or even manganese. In silicate-based molten oxides, the list extends to silicon. However, the development of electrolysis in molten oxide for more reactive metals such as titanium or rare-earth is likely to rely on silica-free melts.
Figure 6. Minimum cell voltage for the isothermal and isobaric decomposition of some pure oxide compounds to metal in equilibrium with 1 atmosphere of oxygen. The oxide and the metal are in their thermodynamically most stable state at the temperature of interest.
Electrolyte theory framework
The development of electrowinning of metals, for example in aqueous electrolyte, has greatly benefited from the availability of powerful and predictive thermodynamic and physical chemistry theories, such as the dilute electrolyte concept and its subsequent amendments. Given the content in each constituent of molten oxides electrolytes presented above, oxygen could be identified as the possible 'solvating species' for the electropositive metallic elements. This approach is unfortunately insufficient for silicates, where both covalent and ionic bonds are governing the molecular entities that are formed and their properties. In that respect, the electronegativity formalization and the rules for cation coordination from Pauling have proven very fruitful to qualitatively determine the covalent or ionic nature of a melt, as well as the nature of the anionic or cationic species that are likely to be formed.15 The electropositive elements, and by extension, their oxides are qualified as Lewis acids (more covalent, network formers, e.g. SiO2) or bases (more ionic, network modifiers, e.g. CaO). Such qualification has since been quantified using concepts like electronegativity or optical basicity.16,17 This approach has been extensively applied for silicates and offers to distinguish various oxygen entities (bridging, non-bridging and free oxygen), very valuable for the theoretical description of the oxygen evolution reaction 1b, see Ref. 18. These concepts have yet to be transposed to systems free of network-formers, alumina, or alkali/alkaline-earth oxides, e.g. for molten oxide systems based on reactive metal oxides.
It is beyond the scope of this review to report the progress in modeling of thermodynamic and physical properties of molten oxides, which can be read elsewhere (for an introduction, see Ref. 14). The existing models are mostly valid for silicate systems and are constrained to narrow ranges of composition and temperature. They are partially helpful for extrapolation while ineffective for system with limited experimental data. Ab-initio calculations have predicted molecular entities in oxide melts that match the structures determined by neutron diffraction or NMR, but this success has again only been achieved for a narrow range of composition with a limited amount of constituent elements [e.g. Refs. 19 and 20]. So far thermodynamic modeling approaches have been more successful by interpolating data based on compounds observed in the solid phase and postulated molecular structures in the liquid [e.g. Refs. 21 and 22].
Perspective
The thermodynamic and physical description of oxide melts based on silica is an on-going challenge due to the high non-ideality of such melts,23 but the abundance of data and the intensive research conducted on those systems have provided means to interpolate their properties. The lack of data for multicomponent systems without network-formers is notorious and severely limits researchers' ability to broaden the range of electrochemical applications of molten oxides. This is a drawback for the development of oxide electrolysis for metals less common and more reactive than silicon, e.g. the rare-earth elements. Part of this challenge is eloquently expressed in Ref. 23: the coulombic nature of melts 'exacerbates the differences in the chemical nature of elements', and leads to difficulties in developing an overarching thermodynamic approach. Ideal concepts such as those developed in other solvents are then of limited value for the molten oxide electrochemist. Unfortunately for molten oxide electrolysis there currently exist only few reliable tools to optimize the electrolyte composition with respect to the transport properties, as detailed in the next section.
Transport properties
The transport properties of the electrolyte are key to the success of a metal extraction electrolytic process. Indeed, the specific energy consumption for the product ( ) is solely determined by the cell voltage (Ucell) and the faradaic efficiency (ϕ, selectivity of the current for reaction 1), with n the number of exchanged electrons and M the molar mass of the metal product:
) is solely determined by the cell voltage (Ucell) and the faradaic efficiency (ϕ, selectivity of the current for reaction 1), with n the number of exchanged electrons and M the molar mass of the metal product:
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/162/1/E13/revision1/jes_162_1_E13eqn6.jpg)
The cell voltage is composed of the thermodynamic minimum (U°, as presented in Figure 6), the kinetic limitations for the cathode and anode reaction (η, e.g. for 1a and 1b) and the ohmic drop (Rohmic·I) as shown in equation 5:
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/162/1/E13/revision1/jes_162_1_E13eqn7.jpg)
The three last terms are the sources of irreversible energy loss. The dominant term is the ohmic drop and is necessary for charge transport across the electrodes and to provide the heat necessary to maintain the process temperature by Joule effect. The electrolyte conductivity and the inter-electrode distance govern the magnitude of Rohm. The distance between the electrodes is the results of the engineering of the cell, and is not discussed in the present review. The electrical conductivity of molten oxides is unusual compared to other electrolytes; and is addressed in the forthcoming subsection.
At high temperature, mass transfer typically controls the rate of production (reaction kinetics are fast); and the productivity of the process is limited by the maximum rate of transport of the reactant to the electrode surface, which determines the limiting current density and in turn the productivity of the cell. This can only be computed with extensive knowledge of the mass transport properties, which are discussed in the second subsection.
Electrical conductivity
Electrical conductivity is one of the physical properties that vary over the largest range across materials, and molten oxides are no exception. A pure molten oxide can exhibit poor conductivity, less than 10−5 ohm−1·cm−1 in the case of a network former, or exceptional conductivity, greater than 102 ohm−1·cm−1 in the case of FeO1-x. In the latter case, the conductivity involves electrons as observed for most multivalent 3d-transition metal oxides.
The electrical conductivity of multicomponent molten oxides, in particular silicates, has been investigated since the early days of usage of electricity for metal processing and has been reviewed extensively e.g. Ref. 24, later Refs. 25, 26, 14, 23, 27, 28. Early investigations of melts' electrical conductivity have proven to be exceptionally valuable with research being conducted outside of just the metallurgy community. For example, 2-electrodes measurements have been used to evaluate an oxide melting point as early as 1934.29 Those methods also provided the first insight into the ionic structure of molten oxides30 at a time when very few in-situ structural observations were available.
In silicates, the electric charge is primarily transported by cations (transference number of unity, Refs. 31 and 32). The cation contribution increases with the basicity and concentration of the network modifiers, as illustrated in Figure 7. Silicate binaries exhibit electrical conduction that is thermally activated with an activation energy barrier nearly half that for viscous flow (between 10 and 20 kcal·mol−1),33,34 though the correlation is not valid for all oxides at any composition. Data for non-silicate molten oxides are scarcer. In agreement with structural viewpoints developed in silicate science, high electrical conductivity has been observed in Al2O3-CaO (up to 1 ohm−1·cm−1). This value can increase by a factor 2 in the presence of ∼20 wt% CaF2.35
Figure 7. Range of electrical conductivity for various binary silicates (molar ratio in parenthesis). Data from.14
As mentioned previously for thermodynamics, the electrolyte theories derived for aqueous solutions are of limited application in oxide melts since there are no true solvating species. The major element, oxygen, more precisely O2−, and its bridging and non-bridging variants, have indeed not been shown to contribute to the charge transport. This is in contrast to the behavior of anions in other mainstream electrolytes. Also intriguing is that the dissociated feedstock oxide (e.g. FeO) becomes the main contributor to the electrical conductivity as its concentration increases, an observation that is in striking difference with most other metal electrowinning electrolyte. Existing predictive tools acknowledge this physical reality by specifically considering the chemical nature of each compound and conducting numerical interpolation from available experimental data.28
Electronic conductivity
The existence of electronic conductivity in molten media is an active area of research for physics, and has been reviewed in some recent publications.36,37 This phenomenon has been acknowledged early,38 notably thanks to the investigation of FeO melts. Several observations suggested the exceptional nature of such raw materials: the persistence of the high electrical conductivity of FeO during melting - in contrast to its fayalite counterpart;29 or the impossibility to electrolytically decompose pure FeO to metal. The measurement of a near-zero transport number of FeO-rich molten oxides later confirmed this observation.39 Similar results have been reported for MnO.40 Remarkably, very few experimental investigations of transport numbers and electronic conductivity have been conducted since then. Only recently have some accurate electrochemical techniques been put forward and applied to silicate and titanate melts.41,37,42 They are based on the analysis of the current transient in a well-controlled current distribution configuration between the electrodes.
The presence of electronic conduction (a feature also present in solid FeO or MnO at high temperature) can potentially reduce the energetic efficiency of an electrolytic process by offering a direct path for electrons to travel from cathode to anode without participating in faradaic reactions at the electrodes. The simultaneous presence of multi-valence cations (e.g. Fe3+ and Fe2+) is presumably responsible for such electronic conduction, as suggested by its variation with changes in oxygen partial pressure.43–45 The initial interpretation of this phenomenon was inherited from solid-state physics and suggested small polaron hopping in molten oxides.46 Recent works in silicates have offered to refine this concept by taking into account the probability of contact between both valence ions. A new model has recently been devised47 and validated for both FeO and MnO in CaO-SiO2 melts.48 An independent approach has suggested the possibility of substitution of free-oxygen from an oxide network by 'caged-electrons' after exposure to reducing conditions,49 forming an 'electride' material. Remarkably, those extra electrons offer a new conduction band and the final material exhibits conductivity close to that of a metal at room temperature. Understanding and subsequently quantifying the electronic conduction mechanism could enable the development of predictive models for electronic conductivity in liquid oxides, ultimately allowing the optimization of the electrolyte composition for electrolysis.
Physico-chemical properties
It is beyond the scope of this review to exhaustively describe the physico-chemical properties of molten oxides, for which an overview has been presented recently18 and extensive coverage can be found in appropriate references.26,25,14,50,51
The properties of some of the binary systems discussed in the previous section are illustrated in Figure 8, where the diameter of the outer circle represents the density and the position indicates the dynamic viscosity. Oxide melts are denser than other typical metal electrowinning media (e.g. 2090 kg·m−3 for aluminum electrolysis at 960°C), and exhibit a high viscosity above 0.1 Pa·s, several orders of magnitude higher than other electrolytes. The surface tension of molten oxides is also notoriously high (typically greater than 300 mN·m−1)14 resulting in issues of electrolyte wetting and creeping on oxide surfaces.
Figure 8. Viscosity (cross, on left in poise or 0.1 Pa.s) and density (inverse of the diameter of the outer circle, scale available in legend) of some key SiO2-MyOz binaries at the given temperature. The ratio of the inner to outer circle area indicates the weight fraction of MyOz. Data from Ref. 14.
The diffusivity of the cations of interest (Fe, Mn, etc...) in molten oxides at temperatures above 1500°C is on the same order of magnitude as in other electrowinning electrolytes (around 10−9 m2·s−1), as discussed as discussed in Ref. 14 or 18. This property, in combination with a high concentration of feedstock cations provides the ability to operate at unprecedented cathode current densities, typically higher than 2 A·cm−2 (see Historical developments section). The diffusivity of the anionic species, oxide anions in particular, is presumably much lower than that of the cations, as discussed in detail in a recent review dedicated to oxygen electrochemistry in molten oxides.18
Perspective
When considering industrial applications, the high electrical conductivity, concentration of metal cation feedstock, and diffusivity are considered assets of molten oxides, and offer to operate with exceptional current densities. The viscosity of molten oxides, while high, can be mitigated thanks to tuning of the melt composition and/or operation with a high superheat. Though one may predict challenges associated with anodic gas evolution at high current densities, such viscosities are typical of pyrometallurgical processes such as glassmaking or metal extraction, where liquid metal and/or gas are commonly handled.
From an electrochemical engineering standpoint however, the lack of physical and chemical information about the molecular entities involved in the transport processes at each electrode and in the bulk of the electrolyte presents a conceptual difficulty. Some fundamental questions remain, in particular when devising electrolysis with multivalent 3d-transition metals:
- what determines the electronic conduction, and is it detrimental to steady-state electrolysis performance?
- what is the distribution of valency at and between the anode and cathode?
- what is the contribution of migration to transport phenomena?
- what is the physical chemistry of electrolytes free of network formers, e.g. electrolytes free of silica for rare-earth or titanium extraction?
These questions challenge the paradigm typically applied for condensed matter; in particular the one concerning electronic conductivity in molten compounds. The answers will require researchers to reconcile various approaches devised in different fields of applied science (physical chemistry, condensed matter physics) and applications (metallurgy, glass making, geology). This effort shall also be conducted with the electrochemical engineering approach in mind, so that the adequate level of understanding of each phenomenon is achieved to predict limiting situations (e.g. high-current density, large concentration of impurities). The existing models for the prediction of transport properties are insufficient, and laboratory experiments with such systems are unfortunately difficult due in large part to material compatibility issues in systems exempt of silica. Innovative experimental set-ups (for example configurations where molten oxides are levitated, i.e. container-less experiments) or advances in the modeling of those systems are two directions that have to be considered. Altogether, the lack of predictive capability of condensed-matter physics for molten systems is currently the main limiting factor for the design of molten oxide electrolytes optimized for high throughput and low energy consumption.
Electrode materials
The practice of using electrodes to process oxides appeared as soon as reliable, large-scale electric power had been mastered.52 For example, as early as 1906,53 Paul Héroult used graphite as electrodes in a commercialized direct current electric arc furnace to melt and perform the reduction of an iron oxide feed.
Cathode
During the electrolysis of molten oxides, the cathode material serves as the host for the deposition of metal via reaction 1a. If the product is liquid and denser than the electrolyte, the cathode makes the cell floor. As in other metallurgical processes, the liquid metal could then be tapped or siphoned periodically from the cell, making the process semi or fully continuous. The challenges for the cathode are the choice of a material and the design of the current collector. Fortunately, the handling of liquid metal product under electrical current flow is practiced daily and at tonnage scale in electric furnaces and other electricity-based metal processes. Depending on the target metal and impurity tolerance, various approaches are possible as exemplified in iron or platinum alloy smelting furnaces.54
At high temperature, and particularly during the first instant of electrolysis, the reactivity of the cathode substrate and the current collector with the electrolyte poses a challenge. Thermodynamics prevents the use of a metal more reactive than the metal target, lest a metallo-thermic reaction occur with the substrate. The use of Ellingham diagrams provides some guidance in materials compatibility, but more delicate thermodynamic consideration is necessary to ultimately control the metal product composition. One possible approach is described in Ref. 55. Another concern is the alloying of the cathode substrate with the metal product during electrolysis, which may 1) lead to the dissolution of the substrate into the product or 2) change the thermodynamic conditions driving the deposition of the target metal. Both phenomena may operate in concert, beneficial or detrimental to the operation, depending on the thermodynamic properties of the corresponding alloy.
In the laboratory, the cathode substrate is chosen primarily for its high melting point. Typical substrates are carbon or a carbide,56 iron,31 molybdenum,57 and platinum-rhodium alloys.58 Electrodeposition on carbon leads to the formation of metal carbide. In the absence of polarization, both carbon and carbides spontaneously react with the metal ion feedstock in the melts (carbothermic reduction), and as such are often unsuitable cathode materials. Metal substrates are often selected because of their high electrical conductivity and relative resistance to oxidation, but their alloying with the target metal leads to complete or partial melting of the cathode during electrolysis, a clear limitation for the production of metal or the investigation of the deposition reaction.59
Anode
The anode materials challenge for molten oxide electrolysis is comparable to the one faced for aluminum production in molten cryolite electrolyte.60 Indeed no materials are solid, stable, and exhibit acceptable electronic conductivity at the temperature foreseen. All of these properties are desired in an anode used for oxygen evolution following reaction 1b. The anode in these conditions is no longer a monolithic piece of a pure material. Rather the interaction with the electrolyte at high temperature, the evolution of oxygen, and the electric field near its surface lead to a new, stable, material assembly. As of recently, only four material substrates have been satisfactorily operated in laboratory cells: graphite, platinum, iridium and chromium.3,57,61 Graphite is clearly not satisfactory in the perspective of mitigating CO2 emissions. No ceramic materials have found useful application, despite some hints at possible options, see Refs. 39 and 62, then later Ref. 3. Noble metal alloys are commonly used and have been operated at larger scale.63 They have limited long-term performances in most basic melts61 and a clear cost penalty when considering commodity metals production such as iron. Only recently a less expensive chromium-based alloy has proven to be of potential interest at the laboratory scale, confirming the viability of the molten oxide electrolysis (MOE) concept for ironmaking.64 Importantly, the work points to less expensive alloy compositions and novel design strategies. The study demonstrated that refractory oxide layers could be obtained in-situ on core alloys, and that the adaptation of electrolyte composition as well as the current density could allow beneficial conditions for such layers' stability. In particular, the role of migration of the electrolyte components (Al3+ and Ca2+ in that specific study) appeared to be critical to determine the local composition near the anode where the electrolyte composition was shown to be different from the bulk and cathode regions. The reader is invited to consult some of the recent papers in the field for more in-depth analysis.61,64
Perspective
Experimental challenges in the laboratory - such as current collector compatibility with the metal host, resistance to oxidation, difficult management of steep temperature gradient, difficulty in containing a metal pool - have prevented a systematic investigation of both the cathode and anode reactions. The role of the cathode substrate and the metal pool composition on the electrodeposition efficiency and metal purity is a pending question. The long-time performance of anode materials for oxygen evolution has not been experimentally validated. Those experimental challenges are easier to handle at larger scale; where temperature gradients are more readily engineered. This small-scale difficulty is an obstacle for demonstrating the performance of the deposition process, as well as accelerating scientific developments via experimentation. The absence of large-scale molten oxide electrolysis cells in operation for steady-state metal production can be seen as a key bottleneck for the faster development of the technology. This should however not be perceived as a fundamental flaw for the foreseen industrial application since high-temperature metal production and gas evolution in molten oxides has proven to be viable for tonnage production (see Historical developments section).
From a fundamental electrochemistry standpoint, there are few reliable and systematic laboratory data about the cathode reaction 1a; and some key questions remain:
- what is liquid metal electrodeposition from an oxide melt into a metal host? e.g. How does one define 'overvoltage' when the composition of the cathode host and the adjacent electrolyte is likely to change rapidly during the process?
- what is the influence of the metal pool depth on interfacial phenomena?
- what is the actual limiting current density due to mass-transfer limitation in the electrolyte?
- what metal purity can be achieved? e.g. What is the activity of each constituent in the electrolyte and/or in the cathode and how does this lead to co-deposition?
Thermodynamics and physical chemistry data for silicate-based systems allow one to consider using multi-physics modeling to partly answer these questions,65 but the conclusions will be difficult to validate in laboratory cells. Furthermore, if silica-free electrolytes are considered, the lack of data will prevent any conventional modeling approach.
The anode material challenge for oxygen evolution remains considerable. The absence of a fundamental description of oxygen chemistry and electrochemistry in molten oxides forces the researcher to make design choices based on dry-oxidation theories, an approach that lacks certain relevance to the physical situation. Fortunately, some recent experimental results64 in the laboratory have provided a new path for materials design for a given electrolyte composition and metal system. Those results call for a better understanding of the electrochemical engineering of the oxygen evolution in molten oxides, in particular, quantification of electromigration phenomena and concentration gradients close to the anode surface. Such plurality of phenomena are unique to electrochemistry, and appear to be key in determining the success or failure of a given anode material. Clearly, the selection of an anode material is also tied to the metal being produced, since this last governs the choice of the supporting electrolyte. Unfortunately the field currently does not have methods to translate results from one molten oxides composition to another.
Historical Developments and Applications
Physicists' and chemists' fundamental interest in oxides electrolysis appeared as early as electricity became available at significant scale. In 1834, Michael Faraday reported:66 'Many bodies are decomposed directly by the electric current, their elements being set free: these I propose to call electrolytes'. He confirmed that oxides were among those bodies,67 though the decomposition of some oxides, for example lead (PbO, then borate of lead, with a Pt anode, §799) and antimony (SbO, but with formation of Sb2O3 which led to the solidification of the electrolyte, §801) was attained 'with great difficulty'. The forthcoming summary of more recent literature shows that only modest practical progresses have been realized since that time, primarily because of the lack of focus on metal production by molten oxide electrolysis (most likely due to the physical and chemical complexity of molten oxides emphasized in the previous sections).
The steelmaking slag time
Martin published a review of early 20th century successes with molten oxides electrolytes in 1943.24 At that time, the object of electrochemistry was to identify the ionic nature of blast-furnace slags (molten oxides containing impurities from the ore and fluxes). The reader is invited to consult the original publication for the complete list of references. His review describes ideas put forth as early as 1906, when Aiken was granted a patent in the United States for the continuous production of iron from a molten slag. The slag was comprised of silica, calcia, and magnesia in order to operate with a fully molten electrolyte. In 1910, Beckman was granted a patent to 'plate out' iron from a CaO-Fe2O3 melt. Finally, in 1924 Farup et al. demonstrated the refining of an iron anode in CaO-Al2O3-SiO2. By 1930, most of the concepts underlying molten oxide electrolysis to produce metal had been advocated and experimentally reduced to practice. In 1927, Urbain presented the results from Andrieux at the Académie des Sciences in Paris.68 He reported the production of lead, zinc, and cadmium in their liquid state, and molybdenum and tungsten in their solid state from borates with a small addition of fluorides. He interestingly reported the formation of metal borides at low current densities.
In 1943, with the aim to provide a more thorough insight into the ionic nature of slags, Martin demonstrated the production of a gas (most likely CO) on a carbon anode from silicates. He also reported the possible enrichment of the anode location with silica while the cathode area was enriched in CaO. He attributed the electrical charge transport across the cell to the movement of iron and calcium ions without dismissing the existence of neutral species. Subsequent researchers reported the difficulty of producing iron metal in the presence of a large concentration of FeO,38 likely due to electronic conductivity of the melts.
1955 saw two breakthroughs for MOE: platinum electrodes were reported as the first 'inert' anodes for oxygen generation in these electrolytes,39 and the possibility to form SiO2 or magnetite on the anodic side of the electrolysis cell was reported. In 1966, Dancy,32 observed dismutation of ferrous ions (Fe2+) to iron metal and ferric ions (Fe3+) at the top of a CaO-FeO electrolyte free of silica. The lower temperature located at the top part of the experimental set-up was deemed responsible for this reaction. This was one of the few reports that highlighted the difficulty in designing an appropriate electrolyte for iron production, as well as the possible issue of calcium deposition from calcia-based electrolytes at an operating temperature greater than calcium's boiling point (1484°C).
Manganese
The cornerstone silicate-science papers from Bockris30,69 reported successful production of manganese from electrolysis of MnO-SiO2 binaries, as well as the production of oxygen from Li2O- SiO2. Interestingly, those reports also mentioned the possible carbothermic reduction of manganese ions by the carbon anode in the absence of polarization, an issue that has rarely been emphasized for molten oxide electrolysis. In 1966, Winand from the Université of Bruxelles in Belgium was granted patents in Europe and the USA70 for manganese production by electrolysis in molten oxides. His design was built opposing the idea of using molten fluorides and their 'well established disagreements', and instead identified the use of CaO-MgO-Al2O3-SiO2 as a supporting electrolyte for the dissolution of MnO feedstock. His approach relied on the successful performance of a carbon anode. He reported reduction to practice in the laboratory for ferro-manganese production with carbon content below 0.04%, and iron and silicon content up to 2% and 11%, respectively. The current density was as high as 3.5 A·cm−2 and the faradaic efficiency reached 70% on the cathode. Such successful results lead to the industrial application of the technique in Ghent (Belgium) at Sadacem. The results of this operation were published in 1977, presumably several years after the discovery.71 The process was remarkable: an industrial cell of 50,000 A capacity was operated with a specific electricity consumption around 6000 kWh·tMn−1, with 100% recovery of the manganese content, a carbon content in the metal of less than 0.1%, and a current efficiency of 50%. With such successful industrial implementation in place, Winand's group stayed at the forefront of understanding manganese electrochemistry in silicate slags and published pioneering papers focused on electrolytes' electrical properties. 59,72,73 His group mentioned the difficulty in investigating the fundamentals of metal electrodeposition at the laboratory scale.73,59 The group's focus was on the electrochemistry of manganese ion (Mn2+) oxidation- a reaction identified as mass-transfer controlled in the available experimental cell. In two reviews,74,75 Winand later provided a comprehensive and visionary case for molten oxides as electrolytes and emphasized their value for metallurgy. Interest in molten oxides electrolysis for manganese was reborn in 1995 when researchers investigated the effect of direct current in the electric furnace production of ferro-manganese.76 Their findings confirmed the remarkable suitability of molten electrolytes for manganese extraction.
Other metals
The second half of the 20th century saw some important developments related to molten oxides particularly in the USSR where researchers were interested in the ionic nature of melts as well as their potential use for silicon and titanium production.77,78 The feasibility of silicon production was suggested by Dodero79 and Lindstrom,80 and confirmed in the early 80's by DeMattei81 in electrolysis cells on the scale of several amperes. DeMattei reported that a barium oxide-fluorides electrolyte was the only satisfactory candidate. The faradaic efficiency was limited to around 40%, a low figure attributed to the presence of electronic conduction caused by carbon detachment from the anode. An alternate explanation offered was the partial reduction of Si+4 to SiO. Interestingly DeMattei reported that the faradaic efficiency was very dependent on the cell geometry. This observation seems particularly important: if electronic conduction occurs via the movement and transport of 'solvated' electrons, then fluid flow and possible inhomogeneity in composition may be engineered. For example, the different anodic and cathodic environments - and possibly local current density - could be tuned to enhance or suppress electron transport.
Sadoway later reported extraction of titanium from molten oxides.82 Several authors reported that nickel, cobalt, zinc, and lead were electro-deposited, more precisely alloyed, on a platinum cathode in diopside, alkali-silicate, and borates at low temperature,83–85 though the focus then was not on metal electrodeposition.
Earth and planetary science interest
Remarkably, perhaps due to the importance of reaction 1 in our planet's history, some of the most advanced insights into molten oxides electrochemistry or electrolysis have come from geologists and planetary scientists. First, electrochemistry in molten oxides offers an exceptional probe for the physicochemical properties of the most abundant compounds in the earth's crust under the extreme conditions encountered in this field. Second, the opportunity to generate oxygen from oxide-rich regoliths encountered during outer space exploration makes electrolysis very appealing.86
Oppenheim87,88 investigated the possibility of an 'electrolytic effect' in magma (basalt). According to Lyell,'subterranean currents could possess the decomposing power of the voltaic pile'. Oppenheim conducted his investigation with an unusual set-up. He used a line optical heater, operated without a container and directly observed the molten oxides during electrolysis. His studies, from a practical standpoint, showed that oxygen was generated at the anode and that iron could be produced, but ultimately dismissed the possible existence of such phenomena in the earth mantle.
The extraterrestrial exploration programs renewed the interest of geologists for oxide electrolysis. At the Bureau of Mines, Kesterke89 reported successful oxygen extraction starting from a surrogate of lunar materials - primarily silicates chemically similar to basalts - using an iridium anode. The report stressed that the addition of fluorides to the electrolyte was mandatory to decrease the melting point. A small pilot (15 kW - 100A) was operated with a SiC cathode and could electrolytically produce an outlet stream of ∼7 vol% of oxygen starting from around 1kg of material.
Electrochemical methods have been used extensively by geologists to probe the concentration and chemistry of metal cations.80,83,85 Those results confirmed the production of metals as well as oxygen on platinum wire electrodes. In 1992, Haskin et al.90 publish a conceptual paper that describes the use of molten diopside (CaMgSi2O6) with addition of FeO to produce metallic elements. The origin of the anodic oxygen production remained uncertain to the authors. They advocated the possible re-oxidation of Fe2+ as a key mechanism.
Sustainable metal extraction?
In the early 1990's, Sadoway advocated the use of electrochemical techniques for environmentally respectful metal extraction, and in particular, evoked the idea of using a reactor similar to a Hall-Héroult cell that would operate with molten oxides.91 At that time the term 'pyroelectrolysis' was used, which was abandoned later for Molten Oxide Electrolysis (MOE). Sadoway disclosed the underlying concepts in 3 patents92–94 that stressed the need to operate in self-heated mode as well as described a possible path toward the use of an inert or consumable anode material. The application of MOE for the GHG-free production of uranium, titanium, nickel, chromium (stainless steel components), and even iron was considered.91,95 This last idea attracted the steel industry which embrace the technology in the perspective of mitigating GHG emissions.5
Subsequent reports mentioned the ability to process chromate wastes using molten oxides with the goal of reducing Cr6+ to Cr3+.95,96 Experimental investigation of oxygen evolution from lunar regolith surrogate was reported in the early 2000s, when Ir and Ir-W anodes were operated.97 Issues such as steady-state current efficiency in the presence of multivalent transition metal ions were emphasized in such relatively large scale experiments (5 A to 10 A).97,98,99 Typical faradaic efficiencies with the selected electrolytes amounted to 70–90%, although they clearly decreased with electrolysis time.
In 2011, Wang57 reported the production of oxygen and iron, both liquid and alloyed with the molybdenum substrate cathode, in laboratory cells fitted with an iridium anode. Later reports demonstrated the influence of electrolyte composition on iridium anode stability61 which proved to be limited in the most basic electrolytes. In 2013, the possibility to operate with refractory-metal based alloys such as chromium as the anode material substrate was demonstrated along with the possibility to achieve exceptionally low carbon content in the iron product.64 This report revealed that a refractory layer composed of a solid solution of Cr2O3 and Al2O3 could be stabilized on the core alloy in a laboratory cell for a time-scale of several hours. This layer exhibited sufficient electrical conductivity to allow oxygen evolution with acceptable ohmic drop increase due to the oxide layer; and was formed thanks to the simultaneous presence of the electrolyte, the generated gas, and the electric field. Key limitations of such studies have been the lifetime of the crucible (6 hours) and the difficulty of assessing faradaic efficiency in the laboratory cells. Nevertheless, those results allow, at least conceptually, a broader choice of materials that are more affordable and available than the classic noble metals used since the discovery of molten oxide electrolysis.
Acknowledgments
Two things make the developments of such innovative technology possible: people and funding organizations. On the first aspect, the author wishes to thank Jean-Pierre Birat, Hervé Lavelaine de Maubeuge and Donald Sadoway with whom he had the honour and privilege to work. On the second aspect, the author acknowledge the pioneer initiatives and financial support of the European Research Fund for Coal & Steel, ArcelorMittal, the American Iron & Steel Institute, the US Department of Energy and the Office of Naval Research.
Footnotes
- a
The choice of a divalent feed is purely didactic since actual ores have higher valency. The challenge related to multivalent oxide feedstock in molten oxides is addressed in a later section on electrical properties.
- b
The present section does not discuss the engineering of heat generation (e.g. thermoneutral voltage approach) to maintain the reactor at constant temperature, as presented in Figure 2a.