Abstract
The reactivity of lithiated graphite and deintercalated LiNi1/3Mn1/3Co1/3O2 (called NMC) in electrolytes containing triphenyl phosphate (TPP) was studied by Accelerating Rate Calorimetry (ARC). Lithiated graphite shows similar reactivity in electrolytes containing TPP to those without TPP. By contrast, deintercalated NMC shows an additional exothermic reaction at lower temperature (∼ 200°C) in electrolytes containing TPP compared to electrolytes without TPP. This means charged NMC shows a higher reactivity at a lower temperature when TPP is present. The flame retardance of electrolytes as a function of TPP content was judged using self-extinguishing (SET) measurements. The SET results show that TPP can significantly reduce the self–extinguishing time of the electrolyte, which may lead to a less-flammable electrolyte. However, the SET test results depend heavily on the test method used. Based on these fundamental results, it is not clear if additions of TPP will actually lead to safer Li-ion cells. Abuse testing experiments on large size Li-ion cells are required.
Export citation and abstract BibTeX RIS
Lithium-ion batteries are used in portable electronic devices, power tools and electric vehicles due to their high energy density and long cycle life.1,2 In rare cases, safety incidents with Li-ion batteries can lead to cases of fire. Fire is caused by two factors: 1) the reactivity of the charged electrode materials with the electrolyte at elevated temperatures and 2) combustion of the organic electrolyte solvents released from the cell.3,4
Non-flammable electrolytes containing flame retardant additives (FRs) have been proposed as one means of decreasing the risk of Li-ion battery fires.5–11 Xu et al. compared the properties of trimethyl phosphate (TMP), triethyl phosphate (TEP) and hexamethoxycyclotriphosphazene (HMPN) as flame retardants in lithium-ion battery electrolyte. They found that these phosphorus compounds imparted a limited reduction in flammability, were unstable on the anode surface and had a relatively high cost. But they also pointed out it was possible to modify the structure of phosphorus alkyl esters or phosphazenes to improve their properties.7 Hu et al. studied bis(N,N-diethyl)(2-methoxyethoxy)methylphosphonamidate (DEMEMPA) as a flame retardant additive for lithium-ion batteries. They found that adding 10% DEMEMPA could make a non-flammable electrolyte but the compatibility between the electrolyte and natural graphite became worse.11 Triphenyl phosphate (TPP) has been shown to be a promising flame retardant additive in studies by Smart et al. and Amine et al.12,13,14 However these researchers did not report studies of electrolytes containing contents of TPP as high as 40%, which may be necessary to actually impart flame retardant properties.
Recently, our group reported the impact of triphenyl phosphate (TPP) as a flame retardant additive on the electrochemical performance of both positive and negative electrode materials using studies involving symmetric cells, automated storage tests and AC impedance spectroscopy.15,16 The work included studies of TPP contents from 0 to 40% by volume in the electrolyte. It was found that a more stable SEI on the negative electrode material was formed in the presence of TPP but that this film had larger impedance.15 Similarly the presence of TPP was found to strongly increase the impedance of positive electrode symmetric cells with either Li[Ni0.8Co0.15Al0.05]O2 (NCA) or NMC electrodes.16 In the case of graphite/graphite and NCA/NCA symmetric cells, charge-discharge capacity retention in the presence of TPP equivalent to that in the absence of TPP was observed. In the case of the NCA/NCA symmetric cells, 2% vinylene carbonate, was required to achieve acceptable performance of cells with 30 or 40% TPP by volume.16 The NMC/NMC symmetric cells showed inferior performance compared to the NCA/NCA cells in the absence and presence of TPP for reasons that we do not yet understand.
The results in References 15 and 16 as well as work by Smart et al.12,13 suggest that it may be possible to make Li-ion cells with up to 40% TPP that function adequately. The major remaining question is whether such cells would provide a safety advantage over cells without TPP. The answer to such a question must involve studies of the reactivity between the charged electrode materials and TPP-containing electrolyte and studies to determine if TPP does impart flame retardant properties to the electrolyte.
In this paper, the performances of NMC/NMC and graphite/graphite symmetric cells containing TPP previously published are first summarized. The reactivity of lithiated graphite and deintercalated NMC in electrolytes containing different amounts of TPP were studied using accelerating rate calorimetry (ARC). NMC was selected for this work instead of NCA because charged NMC has been shown to be less reactive with electrolyte than charged NCA.17 The flame retardant properties of electrolytes containing TPP was judged by self-extinguishing time (SET) tests. These results are an appropriate supplement and continuation of our previous work on the impact of TPP on the electrochemical properties of both positive and negative electrode materials.15,16
Experimental
NMC and graphite (mesocarbon microspheres heat treated to near 2650°C) were obtained from 3M Company and E-One Moli Energy Canada, respectively. The active material (96% by mass for NMC/93% for graphite), Super-S carbon black (2%), polyvinylidene difluoride (PVDF, 2% for NMC/5% for graphite) and some N-methyl pyrrolidinone (NMP) were mixed into a slurry which was spread on an aluminum (for NMC)/copper (for graphite) current collector and dried at 120°C (for NMC)/90°C (for graphite) for two hours. The electrodes were compressed by calendar rollers under an actual pressure of 2000 atm. Using a precision punch, the positive electrode strips were punched into 13 mm diameter disks. Then the disks were dried under vacuum at 120°C for at least 6 hours before being assembled into 2325 coin-type cells.
Triphenyl phosphate was purchased from Aldrich (≥99%). All other electrolyte components, including solvents and LiPF6 were obtained from Novolyte Technologies (Cleveland, OH.). The control electrolyte used was 1 M LiPF6 in 33.3% ethylene carbonate: 66.6% diethyl carbonate (EC:DEC 1:2 v/v). Other electrolytes with different concentrations of TPP (10%, 20%, 40% by volume) were prepared and used as follows:
- 10% TPP - 1 M LiPF6 in 30% EC:60% DEC:10% TPP;
- 20% TPP - 1 M LiPF6 in 26.7% EC:53.3% DEC:20% TPP;
- 40%TPP - 1 M LiPF6 in 20%EC:40%DEC:40%TPP.
The NMC/NMC and graphite/graphite symmetric cells were assembled following the procedure described in our previous work.15,16,18 The symmetric cells were then cycled with a constant current between −1.0 V and 1.0 V (for NMC) or between −0.5 V and +0.5 V (for graphite) on a computer controlled battery tester from E-One Moli Energy Canada.
ARC sample preparation methods were the same as reported in our earlier work.19,20 Pellet-electrode coin cells were made and charged to 4.2 V or discharged to 0 V using the protocol described in Reference 19. Then the cells were opened in an argon-filled glove box and the electrode powder was rinsed with DMC four times and dried in the glove box antechamber as described in Reference 20. Figure 1 in Reference 21 shows that the rinsing procedure does not affect the reactivity of the sample with electrolyte at elevated temperature, suggesting that the formed SEI is not significantly perturbed by the rinsing process. Alternatively, if the DMC rinsing does perturb the SEI, then Figure 1 in Reference 21 suggests that the same interface as on the unrinsed electrode must form at elevated temperature during the ARC experiments. Typically, 94 mg charged positive electrode material and 30 mg electrolyte or 70 mg lithiated negative electrode material and 70 mg electrolytes were used for the ARC tests. The ARC starting temperature was set at 70°C (for NMC) or 80°C (for graphite). Exothermic reactions were tracked under adiabatic conditions when the sample self-heating-rate (SHR) exceeded 0.03°C/min. Experiments were stopped at 350°C or when the SHR was higher than 20°C/min.
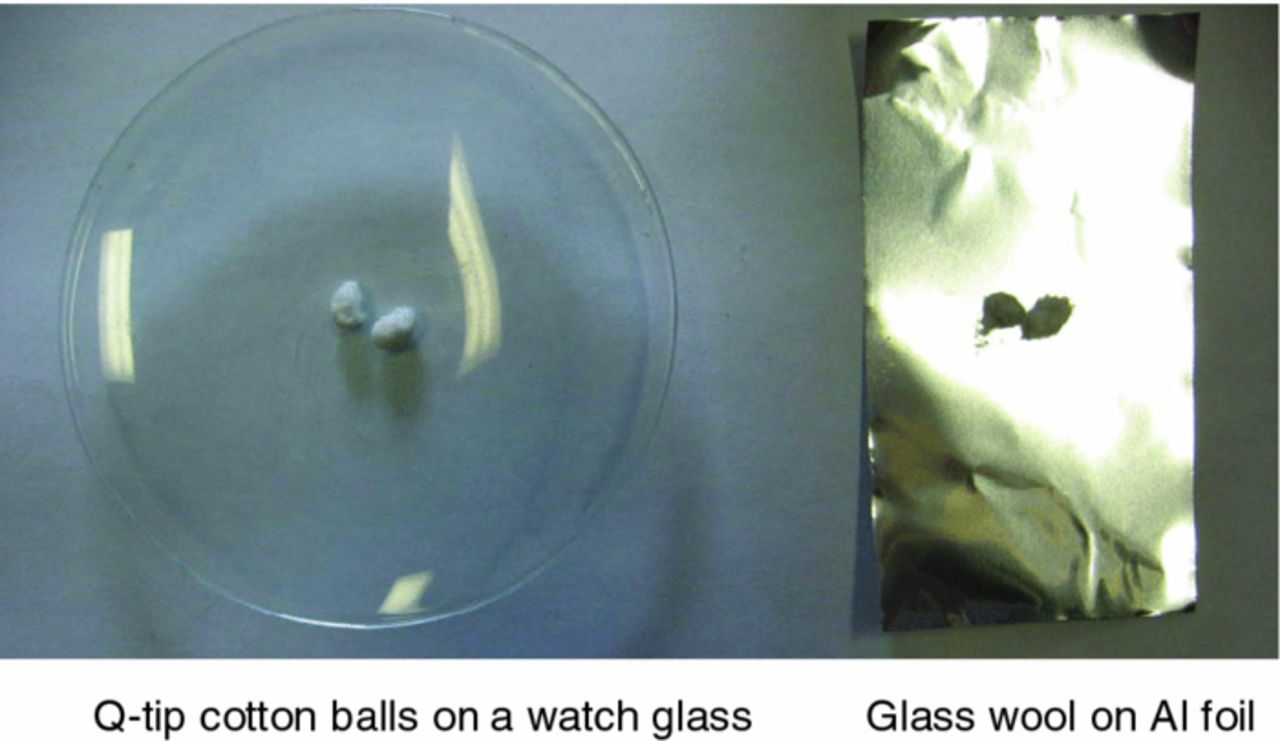
Figure 1. Illustration of the two different SET test methods used in this work: (right panel) Method 1: "Q-tip" tip on a watch glass; (left panel); Method 2: glass wool on Al foil.
Single-point BET specific surface area measurements were made using a Micromeritics Flowsorb II 2300 surface area analyzer. The specific surface areas of NMC and graphite used here were 0.18 m2/g and 0.7 m2/g, respectively.
Self-extinguishing time tests were carried out following the procedure described by Xu et al.7 However, we employed two similar methods to study the method-dependence of these results. In the first method, the cotton tip end of "Q-tips" (Life Brand, Canada) having an ellipsoidal shape about 0.4 cm in diameter and about 0.8 cm long were used. The "q-tip" was placed in a watch glass as shown in the left panel of Figure 1. Figure 1 shows two "q-tip" tips although only one was used in the SET test. These "q-tip" tips had 100 mg of electrolyte added with a syringe. In the second method, a small sphere of glass wool was placed on aluminum foil as shown in the right panel of Figure 1. Figure 1 shows two glass wool spheres although only one was used in the SET test. These glass wool spheres had 100 mg of electrolyte added with a syringe. In both methods 1 and 2, the balls were ignited using a barbeque lighter (Atlantic Superstore, Halifax, N.S.) and the time that it took for the flame to extinguish was recorded. The SET was obtained by dividing the time the flame burned by the electrolyte mass according to the procedure in the literature.7
Results and Discussion
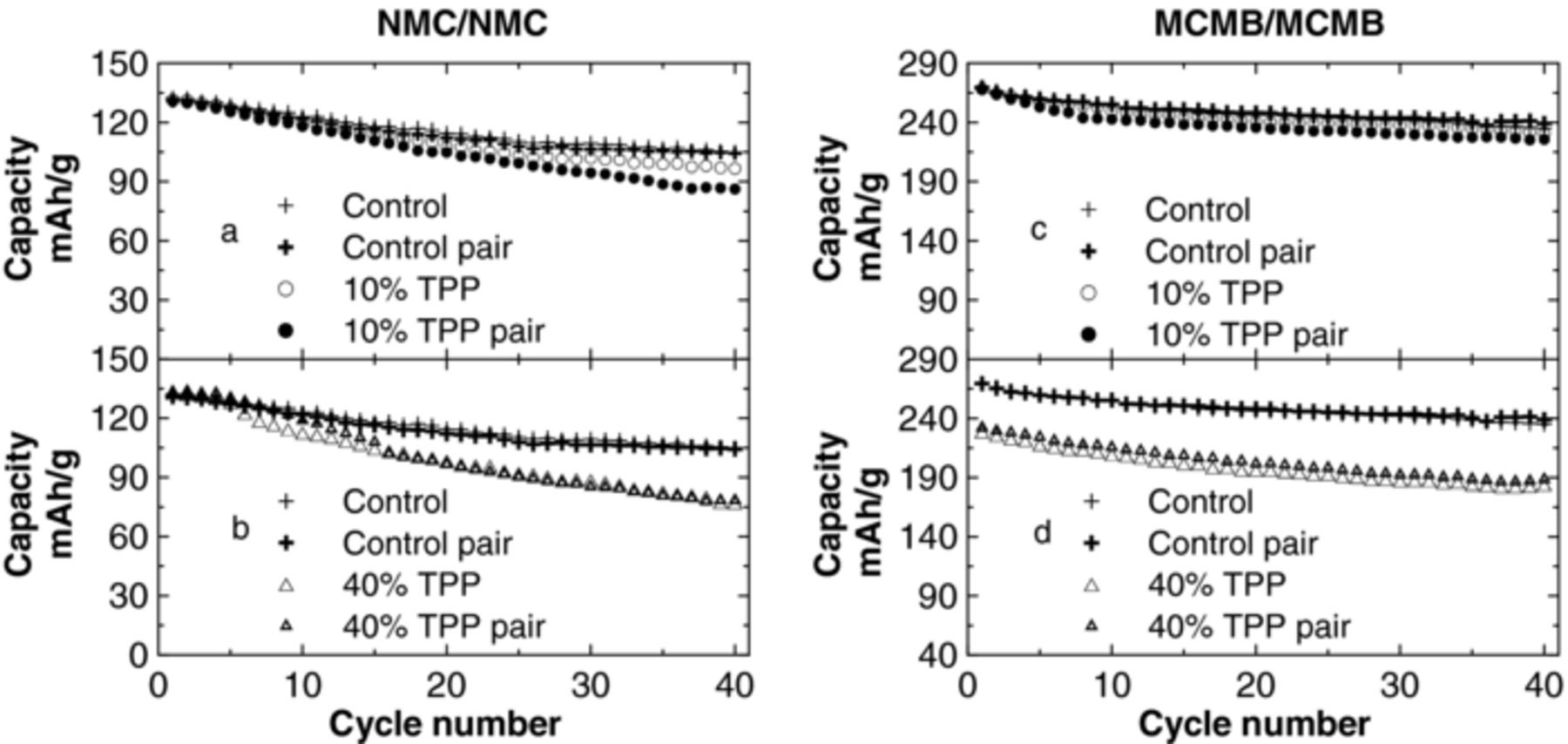
Figure 2 shows the specific capacity versus cycle number for the NMC/NMC and graphite/graphite symmetric cells with 0 (control), 10% and 40% TPP cycling at 30°C ± 0.1°C (duplicate cells are shown). These results have been taken from references 12 and 13 and are included here for the convenience of the reader. TPP increases the capacity fading of NMC/NMC symmetric cells compared to control cells and it also reduces the initial capacity of graphite/graphite symmetric cells compared to control cells. The capacity fading of NMC/NMC symmetric cells and the reduction of the initial capacity of graphite/graphite symmetric cells became more significant as the TPP content increased. These changes are caused primarily by the increased impedance of the surface films caused by the TPP containing electrolyte.15,16
Figure 2. The specific capacity versus cycle number of NMC/NMC and graphite/graphite symmetric cells with different concentrations of TPP in their electrolytes as indicated. The cells were cycled at C/10 at a temperature of 30.0 ± 0.1°C. Results for two cells are shown for each TPP concentration and results for control cells are included in each panel for comparison.
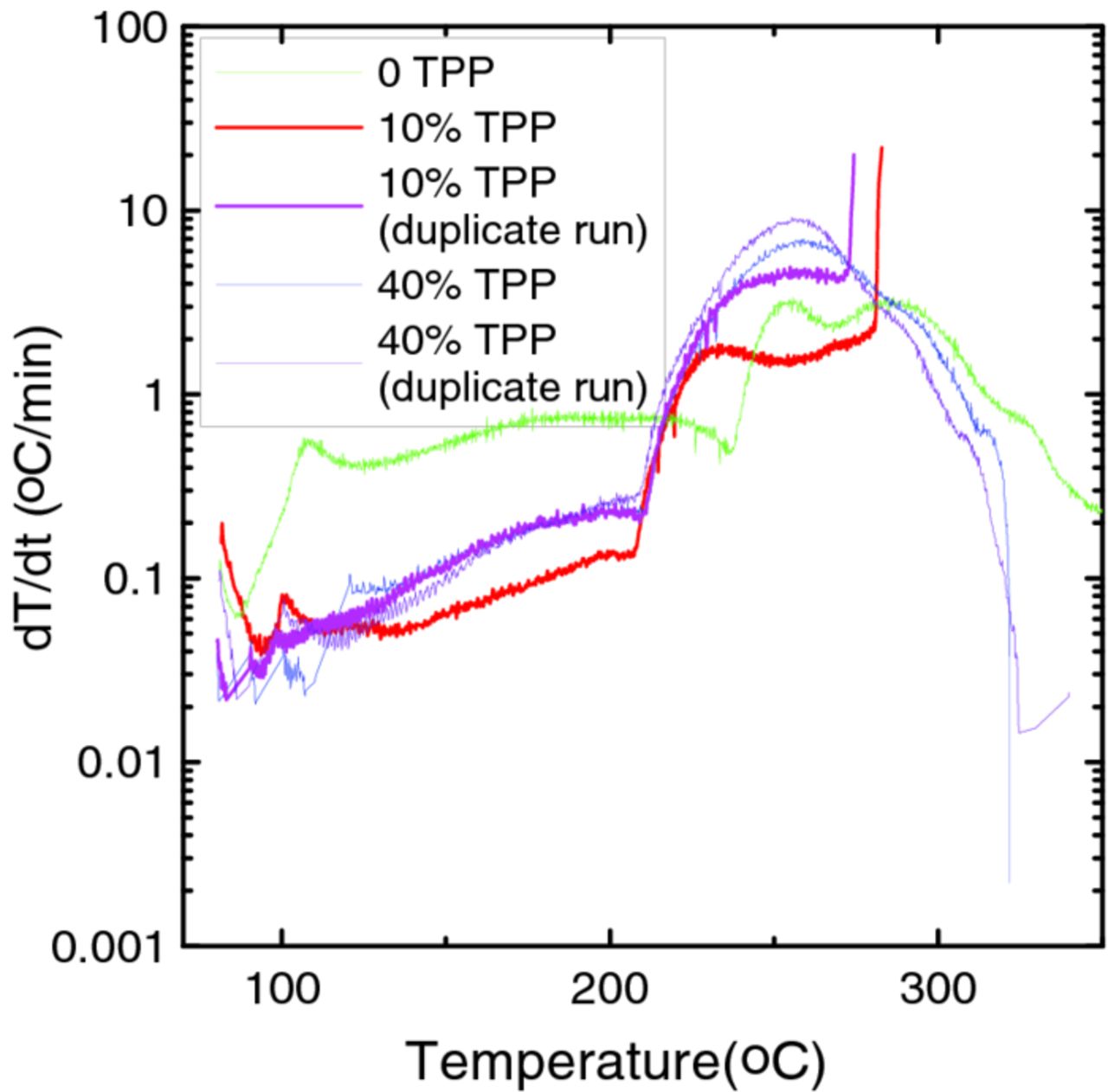
Figure 3 shows the self-heating rate vs. temperature, T, as measured by accelerating rate calorimetry, of 70 mg of LiC6 in the same mass of various electrolytes with different concentrations of TPP. LiC6 shows less reactivity in electrolytes containing 10% TPP or 40% TPP than it does in the control electrolyte in the temperature range between 100 and 200°C. The LiC6 samples with 40% TPP show similar reactivity to the control sample above 200°C as well. This result indicates that adding TPP does not intensify the reactivity of lithiated graphite in electrolyte at elevated temperature.
Figure 3. Self heating rate (SHR) vs. temperature for 70 mg lithiated graphite (LiC6), in the same mass of electrolytes containing different amounts of TPP. The legend indicates which curve corresponds to which sample.
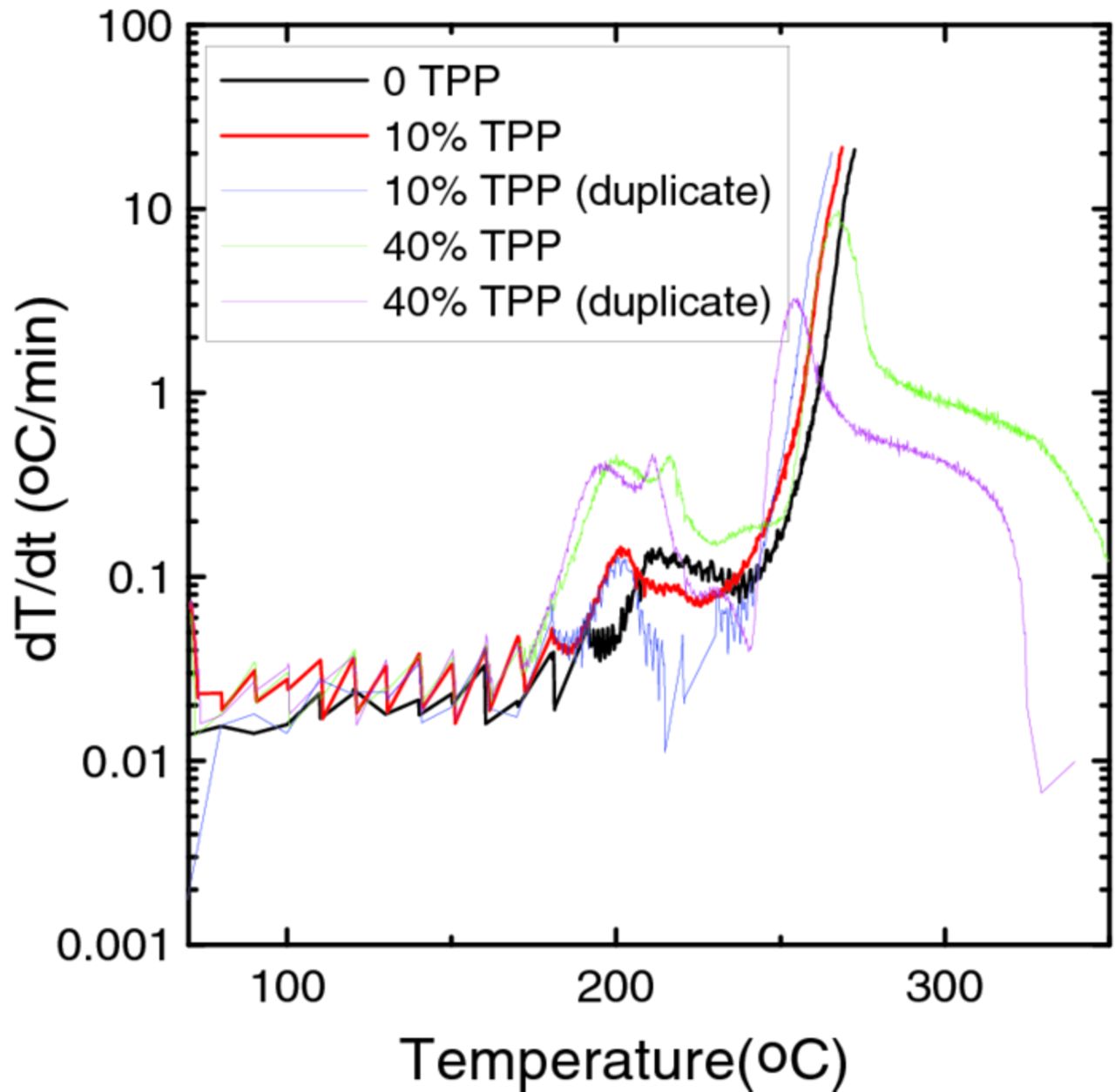
Figure 4 shows the self-heating rate vs. temperature, T, of 94 mg of deintercalated NMC in 30 mg of electrolytes with different concentrations of TPP. Unlike lithiated graphite, charged NMC shows quite different reactivity in electrolyte containing TPP compared to control electrolyte at elevated temperature. A new exothermic peak at around 200°C can be observed in all experiments with TPP-containing electrolytes and the maximum self heating rate of this peak increases with the content of TPP. This indicates a higher reactivity of charged NMC in the presence of TPP. Even though charged NMC does not reach thermal runaway in these experiments with electrolyte containing 40% TPP, the higher reactivity at lower temperature is a serious concern. This means TPP may lead to increased reactivity at the positive electrode side in cells under conditions of electrical or mechanical abuse. Ideally, the addition of a flame retardant would not impact, or would even reduce, the electrode/electrolyte reactivity.
Figure 4. Self heating rate (SHR) vs. temperature of 94 mg of charged NMC (4.2 V vs. LiéLi+), in 30 mg of electrolytes containing different amounts of TPP. The legend indicates which curve corresponds to which sample.
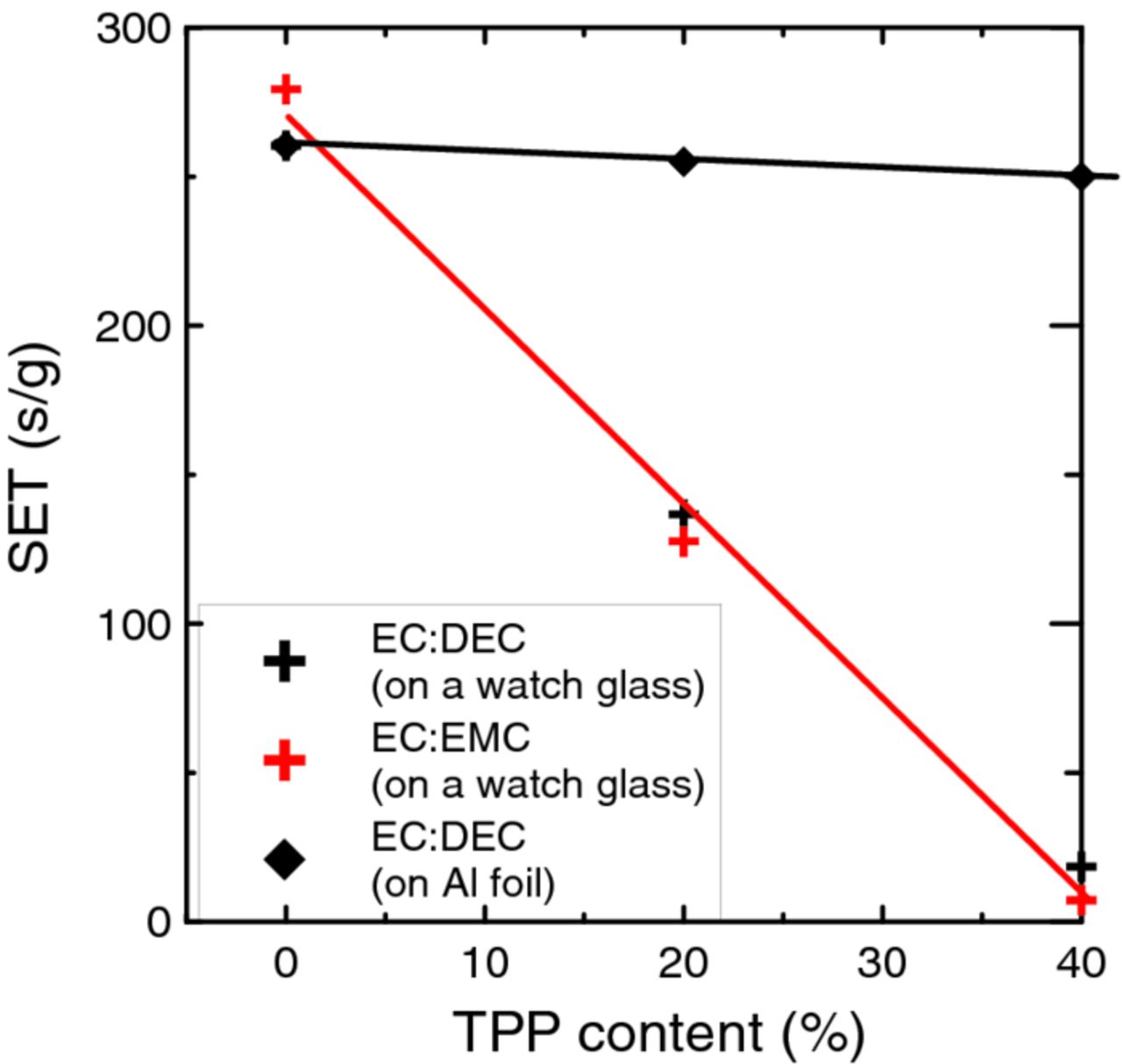
Figure 5 shows the SET plotted vs. the concentration of TPP in electrolytes that use EC:DEC or EC:DMC as solvents. Results from the test methods 1 and 2 described in the experimental section are shown. In each case the SET was calculated by the average of 8 measurements and the error is less than 10% of the plotted value. There are clear differences between the results measured using q-tip tips in a watch glass and glass wool spheres on Al foil. Which of these methods indicates results relevant to the events that occur in a burning Li-ion battery? We do not know and leave this question to the reader. It is worth noting that cotton balls and glass wool were both used in the reported references.7,11 Those references do not report how the cotton balls or glass wool spheres were supported. The SET result using cotton balls on a watch glass shows a nearly linear relationship between the SET and TPP concentration, suggesting that 40% TPP would be very effective as a flame retardant. However, the SET results in Figure 5 measured using glass wool on Al foil give an entirely different conclusion. There is virtually no effect of TPP in reducing the SET. The disagreement between the results measured by methods 1 and 2 strongly suggest that it cannot be guaranteed that TPP will have any flame retardant effect in burning Li-ion batteries.
Figure 5. Self-extinguishing time (SET) for electrolytes containing various concentrations of TPP. Results using the two test methods are shown.
Due to the differences between SET results from methods 1 and 2, small pools of electrolyte on flat surfaces were ignited with the barbeque lighter. The results were not measured quantitatively, but mirrored the results found for the glass wool on Al foil (method 2) where additions of TPP had virtually no effect on the SET. Our research on TPP began due to convincing, but apparently incomplete (based on Figure 5), reports that TPP imparts flame retardant properties to the electrolyte. It is extremely clear that a standard test method to judge the flame retarding effect of electrolytes containing flame retardants needs to be developed and that whatever method is developed must actually have relevance to events that occur in the abuse of Li-ion batteries.
Conclusions
Studies with graphite/graphite, NMC/NMC symmetric cells suggest it may be possible to develop electrolytes containing up to 40% TPP that allow adequately functioning Li-ion cells to be made. The reactivity between lithiated graphite and electrolyte at elevated temperature, as measured by ARC, is no worse in the presence of TPP. By contrast, charged NMC shows a distinctly higher reactivity at temperatures between 180 and 220°C in electrolytes containing TPP than in control electrolyte. These results indicate that adding TPP would not intensify the reactivity of negative electrode material but would make the positive electrode more reactive at lower temperature. We suggest that therefore TPP is not an ideal flame retardant additive.
SET results suggest that TPP is an effective flame retardant using one method and that it is not using another method. The SET test is clearly very method-dependent and almost certainly will not give a valid indication of how a proposed flame retardant will behave in a Li-ion battery abuse scenario. Researchers need to develop methods relevant to Li-ion battery abuse scenarios to judge the impact of proposed flame retardants.
Finally, future publications regarding proposed flame retardants for Li-ion batteries should treat the following topics, at a minimum, to avoid filling the literature with potentially misleading publications:
- (1)Studies of the impact of flame retardants (as a function of concentration) on the negative and positive electrode independently using symmetric cells;
- (2)Studies of the reactivity of flame retardant-containing electrolytes (as a function flame retardant concentration) with charged negative and positive electrode materials;
- (3)Studies using a relevant method (whatever it may be) to determine the flame retardant properties of the electrolyte as a function of flame retardant content;
- (4)If possible (depending on the capability of the research group), studies of cycle testing and abuse testing on full wound Li-ion cells of 18650 (or equivalent) size as a function of flame retardant content.
Acknowledgments
The authors thank NSERC and 3M Canada for funding under the auspices of the Industrial Research Chairs program. P.P. thanks the China Scholarship Council for funding.