Abstract
The reduction products of common lithium salts for lithium ion battery electrolytes, LiPF6, LiBF4, lithium bisoxalato borate (LiBOB), lithium difluorooxalato borate (LiDFOB), and lithium trifluorosulfonylimide (LiTFSI), have been investigated. The solution phase reduction of different lithium salts via reaction with the one electron reducing agent, lithium naphthalenide, results in near quantitative reactions. Analysis of the solution phase and head space gasses suggests that all of the reduction products are precipitated as insoluble solids. The solids obtained through reduction were analyzed with solution NMR, IR-ATR and XPS. All fluorine containing salts generate LiF upon reduction while all oxalate containing salts generate lithium oxalate. In addition, depending upon the salt other species including, LixPFyOz, LixBFy, oligomeric borates, and lithium bis[N-(trifluoromethylsulfonylimino)] trifluoromethanesulfonate are observed.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
A typical lithium-ion battery contains a graphite anode, a lithiated transition metal oxide cathode, and an electrolyte solution composed of inorganic lithium salts dissolved in a mixture of organic carbonate solvents which frequently includes electrolyte additives.1 The long-term cyclability of the lithium-ion battery is dependent upon the anode solid electrolyte interphase (SEI), formed due to the electrochemical reduction of the electrolyte solution.2 Understanding the mechanisms of the reduction reactions along with the products of the reactions is essential for the development of better lithium-ion batteries. The SEI has been proposed to contain lithium alkyl carbonates, lithium carbonate, lithium oxalate, lithium alkoxides, and lithium oxide from the carbonate solvents and LiF, lithium fluorophosphates, lithium fluoroborates, and lithium oxalate from the reduction of electrolyte salts, depending upon the salt utilitzed.3–21 Electrolyte additives have also been used to tailor the properties of the SEI through preferential reduction on anode.1 Despite significant effort over the last two decades, the formation mechanism of the SEI is not well understood. One difficulty in understanding the composition of the SEI is that the SEI is a complicated mixture of compounds, which results from multiple simultaneous and competing reduction reactions. In addition, since the SEI is very thin (∼ 50 nm) and unstable in the presence of oxygen or water, characterization is very difficult. We have reported a detailed analysis of binder free graphitic anodes cycled in simplified electrolytes which suggest that the initial reduction reaction of the carbonates generate lithium alkyl carbonates and LiF as the predominant components of the anode SEI.20,21 Synthesis of initial SEI components from carbonate solvents in high yield through reduction of the solvents with lithium naphthalenide has been reported. The unique advantage of this reduction technique is the generation and isolation of SEI constituents from individual electrolyte components in high yield without competing reduction reactions. Reduction of ethylene carbonate results in generation of lithium ethylene dicarbonate and ethylene, while the reduction of dialkyl carbonates result in lithium alkyl carbonates and alkanes.22 As an expansion of these investigations, the reduction of some of the most common electrolyte salts with lithium naphthalenide has been investigated. All reduction reactions result in precipitation. The precipitates have been analyzed by solution Nuclear Magnetic Resonance (NMR) Spectroscopy, solid-state Infra-Red spectroscopy with Attenuated Total Reflectance (IR-ATR) and X-ray Photoelectron Spectroscopy (XPS). The results provide insight into the formation mechanism of the anode SEI.
Experimental
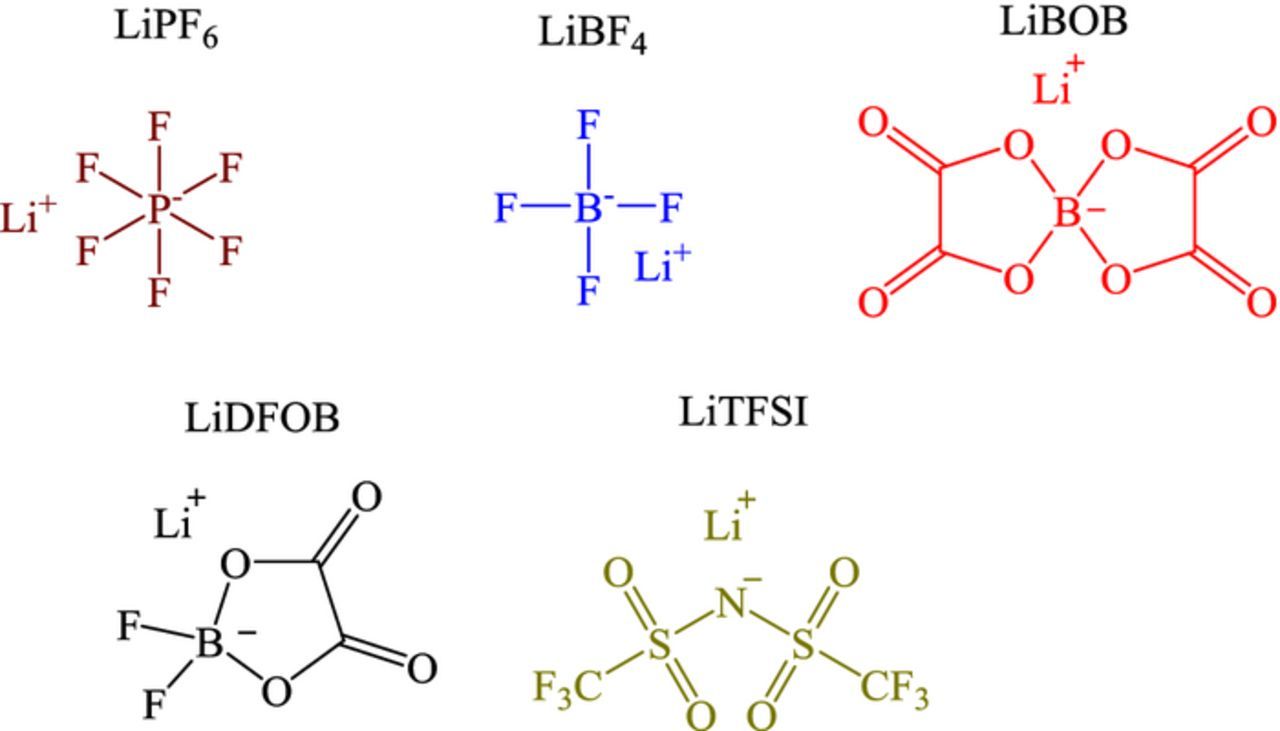
Battery-grade lithium hexafluorophosphate (LiPF6), lithium tetrafluoroborate (LiBF4), lithium bis(oxalato)borate (LiBOB), lithium difluoro(oxalato)borate (LiDFOB), and lithium bis(trifluoromethylsulfonyl)imide (LiTFSI) (Figure 1) were obtained from BASF. Diethyl ether (Et2O), tetrahydrofuran (THF), and naphthalene were purchased from Sigma-Aldrich. Lithium discs were obtained from MTI Corporation. All the reagents were stored in an argon filled glove box at room temperature and used without further purification. Lithium naphthalenide solution (Li[NAP]) in THF or Et2O was prepared with 10 mol% excess naphthalene. Ethereal solvents were used since the solvents do not react with Li[NAP]. The solvent is not expected to significantly alter the reduction products of the salts. Lithium foils were added to naphthalene solution of either THF or Et2O and stirred for 3 days at room temperature. The solution turned green or purple, respectively, in a few minutes after the addition of lithium metal and became darkly colored after stirring for 3 days, as previously reported.23
Figure 1. Structures of the electrolyte salts.
Number of equivalents of reducing agent required for the complete reduction of electrolyte salts was evaluated by NMR analysis. Electrolyte salts were dissolved in either THF or Et2O and reacted with different molar equivalents of Li[NAP], a one electron reducing agent, at room temperature overnight. The resulting reaction mixtures were transferred into clean dry NMR tubes along with a capillary tube filled with deuterated DMSO and an internal standard. The internal standards, LiTFSI; hexafluoro benzene; or LiBF4, were chosen carefully to avoid any overlapping peaks with the starting materials or products. The samples were analyzed with 19F and 11B NMR spectroscopy and the concentrations of the unreacted electrolyte salts were estimated in reference to the internal standard.
Electrolyte salts (LiPF6, LiBF4, LiBOB, LiDFOB, & LiTFSI) were dissolved in Et2O and reduced with appropriate molar equivalents of Li[NAP] in larger scale. The evolved gasses and volatiles in the reaction mixtures were analyzed with GC-MS. The solid residues were washed with Et2O three times, dried overnight at room temperature, and analyzed with IR-ATR, solution NMR and XPS. All the reactions were conducted inside a nitrogen filled glovebox. XPS and IR-ATR analyses were conducted with no exposure to air. NMR, GC-MS were conducted with minimal exposure to air.
GC-MS analyses were conducted on an Agilent 6890-5973N GC equipped with an G973N mass selective detector. Liquid samples were diluted with dichloromethane, mixed with distilled water to remove the residual electrolyte salts and non-volatile inorganic components, and the organic phases were utilized for the analyses. Helium was used as carrier gas at a flow rate of 24 mL/min. The initial column temperature was 40°C and the temperature was ramped at 10°C/min to 200°C and held at that temperature for 2 minutes with the total run time of 18 minutes. The mass spectra obtained were compared to the NIST library to determine their molecular structures. THF, Et2O (solvents) and naphthalene (starting material) and were the only volatile components present in the reaction mixtures. The gas analyses were performed by sampling the head spaces of the reaction mixtures in RB flasks with a 10 μL GC syringe. Helium was used as the carrier gas at a flow rate of 1.5 mL/min. The initial column temperature was set to 40°C, and the temperature was ramped at 1°C/min to 43°C and held at that temperature for 2 min with the total run time of 5 min. The mass spectra obtained were compared to the NIST library to determine their molecular structures.
IR-ATR spectra of the dried solid residues were acquired on a Bruker Tensor 27 spectrometer equipped with a germanium crystal in attenuated total reflectance (IR-ATR) mode. Samples were transferred using air-tight vials and the spectrometer was operated inside a nitrogen filled glovebox to avoid air exposure. Each spectrum was acquired with 128 scans from 700 cm−1 to 4000 cm−1 at the spectral resolution of 4 cm−1. The data were processed and analyzed using the OPUS and Originlab software.
NMR spectra of the samples were collected with a Bruker Avance III 300 MHz NMR spectrometer at room temperature. The solids were dissolved in D2O in the nitrogen filled glovebox and 19F, 31P, 11B, & 13C NMR spectra of the solutions were acquired. 19F NMR spectra are referenced to LiF at −122.0 ppm, and 11B NMR spectra are referenced to residual salts: LiBF4, LiBOB, or LiDFOB at −1.5, 7.4, or 2.9 ppm, respectively. The spectra were processed and analyzed using MestReNova 10.0.2.
XPS spectra of the dried precipitates were acquired using a Thermo Scientific K-alpha XPS. Samples were made into circular pellets with a press or stuck on a conductive carbon tape as a thin layer and transferred from the glovebox to the XPS chamber using a vacuum transfer module without exposure to air. An argon flood gun was used to avoid surface charge accumulation during sample analysis. The binding energy was corrected based on the C 1s of hydrocarbon at 284.8 eV. The data were processed and analyzed using the Thermo Avantage, XPS Peak 4.1 and the Originlab software.
Results and Discussion
Reduction of electrolyte salts
The number of electrons required for the complete reduction of electrolyte salts was investigated by NMR analysis. Electrolyte salts dissolved in either THF or Et2O were reduced with different molar equivalents of Li[NAP] at room temperature overnight. Addition of one molar equivalent of Li[NAP] to LiBOB, LiDFOB and LiTFSI solutions results in immediate discoloration of Li[NAP] and precipitation of solid products, however discoloration in LiPF6 and LiBF4 samples takes roughly an hour, the color change is due to the consumption of Li[NAP] in the reduction of the electrolyte salts. Upon incorporation of higher concentrations of Li[NAP], > 1 molar equivalent, similar discoloration is observed. However, for samples where color retention is observed for more than 24 hours, the quantity of Li[NAP] required to completely reduce the salt has been exceeded, thus allowing determination of the approximate number of equivalents of reducing agent. The reaction mixtures were transferred into NMR tubes and a capillary, filled DMSO-d6 and an internal standard, was added into each tube. The samples were analyzed with 19F and 11B NMR spectroscopy and the concentration of the remaining electrolyte salts were determined via integration of the NMR peaks compared to the internal standard, hexafluoro benzene or LiBF4. Reduction of LiBF4 with 1, 2, and 3 equivalents of Li[NAP] results in consumption of approximately 40, 69, and 96 ± 4% of the LiBF4, respectively, suggesting 3 e− are required for quantitative reduction. Similarly, numbers of equivalents of Li[NAP] required for the reduction of LiBOB, LiDFOB, and LiTFSI were estimated to be 2 e−, 2e−, and 12 e−, respectively. The number of equivalents of Li[NAP] required for complete reduction of LiPF6 could not be measured reliably by NMR spectroscopy. However, in all cases low concentrations of residual salt are observed after the reduction reactions and some of the reduction products may precipitate prior to complete reduction, so the number of electrons required for reduction of the different salts should be viewed as approximate.
The electrolyte salts were then treated with a sufficient quantity of Li[NAP] to fully reduce the salt. All reactions result in a significant quantity of precipitate. The remaining solution was analyzed by GC-MS and NMR spectroscopy. The only component remaining in solution is a low concentration of the unreacted salt. In addition, analysis of the headspace of the samples detected no gaseous products resulting from the reduction reactions. The results suggest that all of the reduction products of the lithium salts are insoluble. Thus, the Li[NAP] reduction of all lithium salts investigated results in quantitative conversion to organic solvent insoluble components.
NMR analysis of the solids
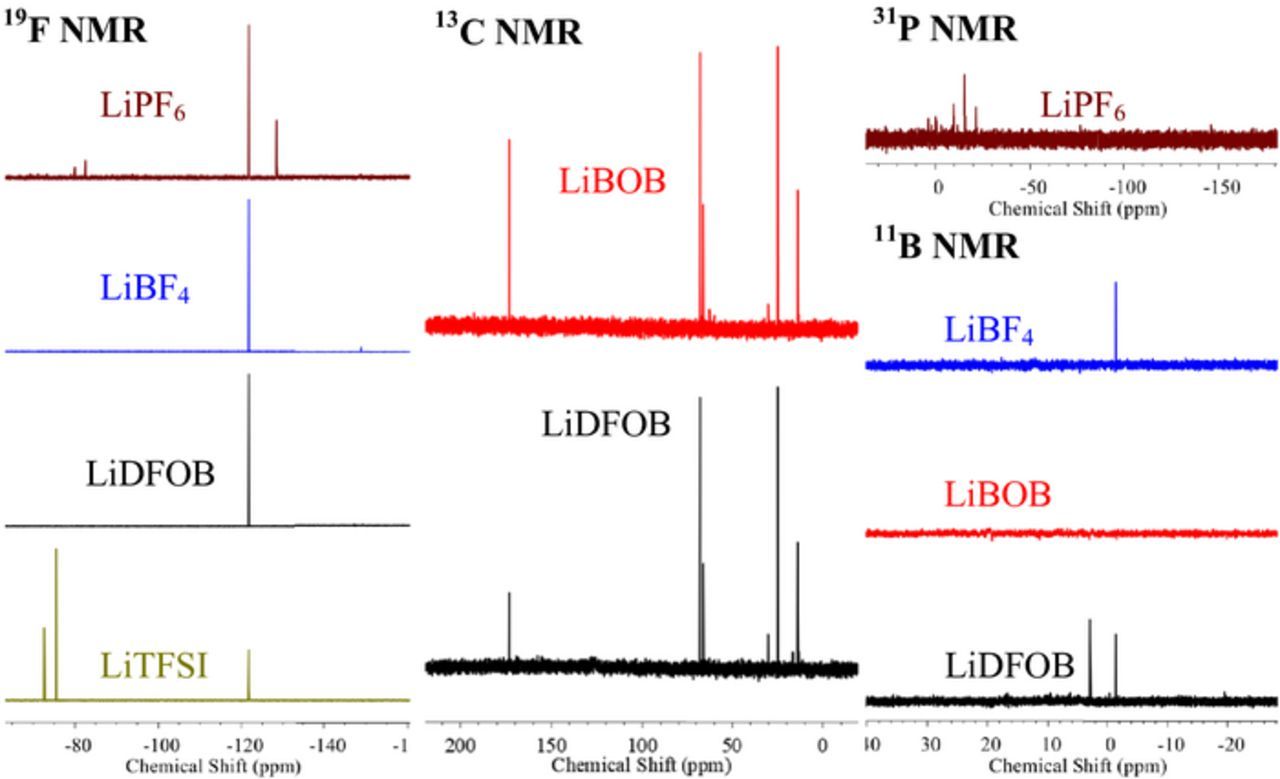
The residual organic solvent insoluble solids have been analyzed via a combination of solution NMR spectroscopy in D2O, Infrared spectroscopy with attenuated total reflectance (IR-ATR), and X-ray photo electron spectroscopy (XPS). The residual solids have been dissolved in D2O for NMR analysis. While most of the residual solids dissolve in D2O, some of the solid does not readily dissolve. In addition, some of the reduction products may react with water to generate subsequent hydrolysis products. The dissolved solids were analyzed via a combination of 11B, 13C, 19F, and 31P NMR spectroscopy. Representative NMR spectra of the solids are provided in Figure 2.
Figure 2. Solution NMR spectra of the solids from the Li[NAP] reduction of electrolyte salts.
The 19F NMR spectrum of the reduction product from LiPF6 displays a strong singlet corresponding to LiF at −122 and a medium singlet at −128.5 ppm corresponding to HF.24 While LiF is a frequently reported as a product of the reduction of LiPF6, HF is most likely generated from the hydrolysis of unreacted LiPF6 in D2O. In addition, a doublet is observed at −81.3 ppm in the 19F NMR spectrum which has a corresponding triplet at −15.7 ppm in the 31P NMR spectrum and a coupling constant of 962 Hz characteristic of LiPO2F2. The presence of LiPO2F2 likely results from the hydrolysis of LiPF2 upon addition of the residual solid to D2O, since no extractable oxygen is present in the reaction media. The XPS data, as discussed below, provides further support for this assignment.
The 19F NMR spectrum of the residual solids from the reduction of LiBF4 contains a strong singlet at −122 ppm characteristic of LiF. In addition, the sample exhibits a weak set of peaks at −149 ppm in the 19F NMR spectrum characteristic of residual LiBF4. A single peak is observed in the 11B NMR spectrum peak at −1.5 ppm characteristic of residual LiBF4.
The 13C NMR spectrum of the residual solids obtained from reduction of LiBOB displays a strong singlet at 173.2 ppm characteristic of in lithium oxalate. The other peaks observed in the 13C NMR spectra are characteristic of residual solvents, THF and Et2O, used for the reduction reaction. The peaks at 67.8 and 25.0 ppm are characteristic of residual THF while the peaks at 66.0 and 14.1 ppm are characteristic of residual Et2O. There are no peaks observed for residual LiBOB in either the 11B or 13C NMR spectra consistent with quantitative reduction of LiBOB under the reaction conditions.
The NMR spectrum of the residual solid from the reduction of LiDFOB is similar to a combination of the reduction products of LiBF4 and LiBOB. The 19F NMR spectrum is dominated by LiF at −122 ppm, but also contains small sets of peaks at −147 and −149 ppm characteristic of residual LiDFOB and LiBF4, respectively. The corresponding peaks characteristic of LiDFOB and LiBF4 are observed in the 11B NMR spectra at 2.9 ppm and −1.5 ppm, respectively. The 13C NMR spectrum contains a strong peak at 173.2 ppm characteristic of lithium oxalate, along with peaks characteristic of residual THF and Et2O. However, unlike LiBOB some residual LiDFOB is observed at 161.1 ppm.
The 19F NMR spectrum of the solids from the reduction of LiTFSI shows a strong singlet corresponding to LiF. In addition, two strong peaks at −75.6 ppm and −72.7 ppm with peak areas in 2:1 ratio. The peak integrations have a 2:1 ratio which is independent of the quantity of Li[NAP] added suggesting that they arise from a single molecular species. The spectral data is consistent with the generation of lithium bis[N-(trifluoromethylsulfonylimino)] trifluoromethanesulfonate (LiOS(CF3)(NSO2CF3)2 as previously reported.25 No residual LiTFSI is observed at −79.4 ppm in the 19F NMR spectrum.
FTIR analysis of the solids
In an effort to further understand the composition of the solids obtained from reduction, the reduction products of the salts have been analyzed with IR-ATR. The IR-ATR spectra of the solids generated from the reduction of LiBOB and LiDFOB are provided in Figure 3. IR-ATR spectra of the residual solids for the other salts were also acquired, but the spectra were dominated by residual solvent and naphthalene since the decomposition products do not contain any functional groups which strongly absorb IR radiation, consistent with the observation of LiF as the predominant component by NMR.
Figure 3. FTIR spectra of the solids from the Li[NAP] reduction of LiBOB and LiDFOB.
The reduction product of LiBOB exhibits strong absorptions around 1670, 1330 and 780 cm−1 characteristic of lithium oxalate. The peaks at 1805 and 1770 cm−1 are characteristic of -CO2-B-CO2- oscillations and the peak at 1250 cm−1 corresponds to combination of O-C-C asymmetric stretching and O-B-O bending, suggesting the presence of a combination of LiBOB and crosslinked oligomeric borates, as previously reported.26,27 A weak broad absorption is also observed between 1400 and 1500 cm−1, consistent with the presence of Li2CO3. In addition to the reduction products, absorptions corresponding to residual THF at 1070 and 910 cm−1 are also observed. The reduction product of LiDFOB displays IR absorptions very similar to the solids from LiBOB consistent with the presence of lithium oxalate, crosslinked oligomeric borates, and Li2CO3, except the intensity of the broad absorption characteristic of Li2CO3 is increased.
X-ray photoelectron spectroscopy of the solids
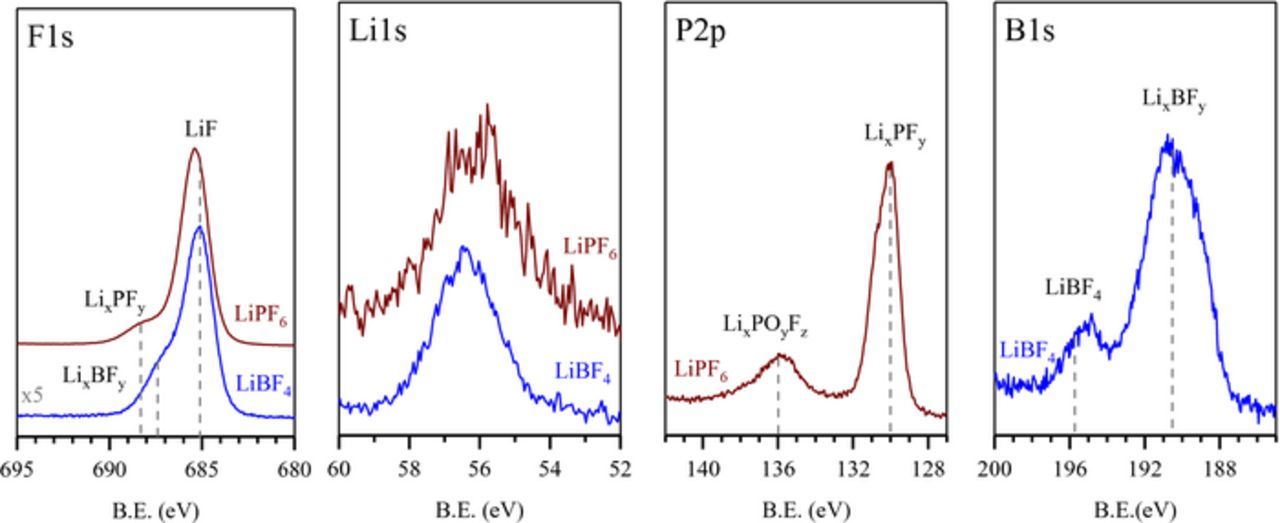
The solids generated from the reduction of LiPF6, and LiBF4 were analyzed with XPS and the spectra are displayed in Figure 4. XPS analysis of the other residual solids was attempted, but the insoluble reduction products contain residual solvent and naphthalene which cannot be removed which resulted in contamination of the XPS analysis chamber for several months. Thus, we were unable to obtain XPS spectra of the other reduction products.
Figure 4. XPS spectra of the solids from the Li[NAP] reduction of LiPF6, LiBF4, and LiTFSI.
The F1s spectrum of the residual solid from the reduction of LiPF6 is dominated by a peak at 685 eV characteristic of LiF. The shoulder at 688.3 eV is characteristic of P-F species in LixPFy and LixPFyOz. The P2p spectrum contains a strong peak at 130.0 eV corresponding to LixPFy species and the small peak at 136.0 eV characteristic of LixPFyOz. The low concentration of LixPFyOz most likely results from reaction of LixPFy with trace oxygen or moisture. The Li1s spectrum exhibits a broad peak around 56.3 eV corresponding to combination of LiF, LixPFy, and LixPFyOz. No residual LiPF6 (F1s, 687.6 eV; P2p, 137.8 eV) is observed.
The F1s spectrum of the residual solids from the reduction of LiBF4 is dominated by a peak at 685 eV characteristic of LiF. A shoulder is observed at 687.5 characteristic of B-F species in LixBFy and residual LiBF4. The B1s spectrum is dominated by a peak at 190.5 eV corresponding to LixBFy species with a small peak at 195.7 eV is characteristic of residual LiBF4. The Li1s spectrum exhibits a broad peak around 56.3 eV corresponding to combination of LiF, residual LiBF4 and LixBFy.
Discussion
The reduction products of some of the most common electrolyte salts have been investigated via a combination of NMR, GC-MS, IR-ATR, and XPS. Upon reduction, all fluorine containing salts generate LiF and all oxalate containing salts generate lithium oxalate, in addition to other components which are dependent upon the structure of the salt. While the proposed equations provide estimates for the stoichiometries of the reactions as obtained from experimental results, due to the insolubility and hydrolytic instability of many of the reduction products quantitative analysis is difficult.
Reduction of LiPF6 yields LiF and LixPFy (Eq. 1). LiF is the predominant species observed by 19F NMR spectroscopy and F1s XPS. The primary phosphorous containing species observed in the P2p XPS spectrum is LixPFy with additional low concentrations of LixPFyOz. The presence of LixPFyOz likely results from the reaction of LixPFy with trace water or oxygen (Eq. 2). Upon preparation of the samples for NMR analysis via dissolution in D2O the LixPFy is converted to LixPOyFz via hydrolysis or oxidation. The reduction products of LiBF4 are very similar to the reduction products of LiPF6. The primary products observed by XPS are LiF and LixBFy. Analysis of the LiBF4 reduction product by solution NMR spectroscopy reveals only LiF suggesting that LixBFy and the LixBFy hydrolysis or oxidation products are not soluble in D2O. The observations are consistent with previous reports on the reduction products of LiPF6 and LiBF4.28,29
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/165/2/A251/revision1/A251equ1.jpeg)
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/165/2/A251/revision1/A251equ2.jpeg)
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/165/2/A251/revision1/A251equ3.jpeg)
The primary reduction product of LiBOB is lithium oxalate although low concentrations of Li2CO3 are also observed by IR spectroscopy (Eq. 4). In addition, crosslinked oligomeric borates are observed consistent with previous reports.30 Reduction of LiDFOB results in the observation of very similar products, lithium oxalate, Li2CO3, and crosslinked oligomeric borates, along with LiF (Eq. 5). While CO2 is not observed by GC-MS analysis for either LiBOB or LiDFOB, the presence of Li2CO3 in the solid residue likely results from CO2 reduction by the excess Li[Nap], as previously reported.31
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/165/2/A251/revision1/A251equ4.jpeg)
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/165/2/A251/revision1/A251equ5.jpeg)
Reduction of LiTFSI results in the generation of two water soluble components, LiF and lithium bis[N-(trifluoromethylsulfonylimino)] trifluoromethanesulfonate as observed by NMR spectroscopy. Lithium bis[N-(trifluoromethylsulfonylimino)] trifluoromethanesulfonate is likely be formed through reductive cleavage of N-S bond, followed by insertion of the lithium imide into the S = O bond (Eqs. 6–7). This mechanism is consistent with a combination of previously reported computational and experimental results.14,15,25 Reduction of LiTFSI requires a large excess of Li[Nap] (∼12) per equivalent of LiTFSI, consistent with further reduction of the intermediate product LiSO2CF3 to a combination of LiF, Li2S, Li2S2O4, Li2SO3, and Li3N (Eq. 8). The presence of these compounds is supported by a previous reports on the reduction products of LiTFSI on negative electrode surfaces.14,32
![Equation ([6])](https://content.cld.iop.org/journals/1945-7111/165/2/A251/revision1/A251equ6.jpeg)
![Equation ([7])](https://content.cld.iop.org/journals/1945-7111/165/2/A251/revision1/A251equ7.jpeg)
![Equation ([8])](https://content.cld.iop.org/journals/1945-7111/165/2/A251/revision1/A251equ8.jpeg)
Conclusions
Reduction reactions of some of the most common lithium salts for lithium-ion battery electrolytes have been investigated. The reduction reactions of LiPF6 and LiBF4 are similar resulting in the generation of LiF and LixPFy and LixBFy, respectively. In carbonate based electrolytes, the LixPFy abstracts oxygen to generate LixPFyOz consistent with previous reports of salt reduction products on anodes in lithium ion batteries. The reduction reactions of LiBOB and LiDFOB are also similar resulting in the generation of lithium oxalate, Li2CO3, and crosslinked oligoborates. In addition, LiF is observed as a reduction product of LiDFOB. Reduction of LiTFSI results in the generation of lithium bis[N-(trifluoromethylsulfonylimino)] trifluoromethanesulfonate along with LiF, Li2O, Li2S, Li2S2O4, Li2SO3, and Li3N.
Acknowledgment
The authors gratefully acknowledge funding from Department of Energy Office of Basic Energy Sciences EPSCoR Implementation award (DE-SC0007074).
ORCID
Brett L. Lucht 0000-0002-4660-0840