Abstract
We have demonstrated the repeated cycling of a redox flow cell based on water-soluble organic redox couples (ORBAT) at high voltage efficiency, coulombic efficiency and power density. These cells were successfully operated with 4,5-dihydroxybenzene-1,3-disulfonic acid (BQDS) at the positive electrode and anthraquinone-2,6-disulfonic acid (AQDS) at the negative electrode. Reduction of the voltage losses arising from mass transport limitations, and understanding of the chemical transformations of BQDS during charging have led to these improvements in performance. The specific advances reported here include the use of organic redox couples in the free-acid form, improvements to the flow field configuration, and novel high-surface-area graphite-felt electrode structures. We have identified various steps in the chemical and electrochemical transformations of BQDS during the first few cycles. We have also confirmed that the crossover of the reactants through the membrane was not significant. The performance improvements and new understanding presented here will hasten the development of ORBAT as an inexpensive and sustainable solution for large-scale electrical energy storage.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
With the increasing penetration of solar photovoltaic and wind-based electricity generation, the variable and intermittent output of these energy generation systems is a grave concern for stable operation of the electricity grid. To buffer the inevitable surges in electricity supply and demand, large-scale energy storage systems are needed. Such energy storage systems must be capable of storing thousands of giga-watt hours of electricity per day. Rechargeable batteries are particularly attractive for electrical energy storage because of their high energy efficiency and scalability.1–3 However, for such a large-scale application, these batteries must be inexpensive, robust, safe, and sustainable. None of today's commercially-available batteries can meet all the performance and cost targets at this scale of deployment of energy storage. This situation has led to a global search for a transformational solution.
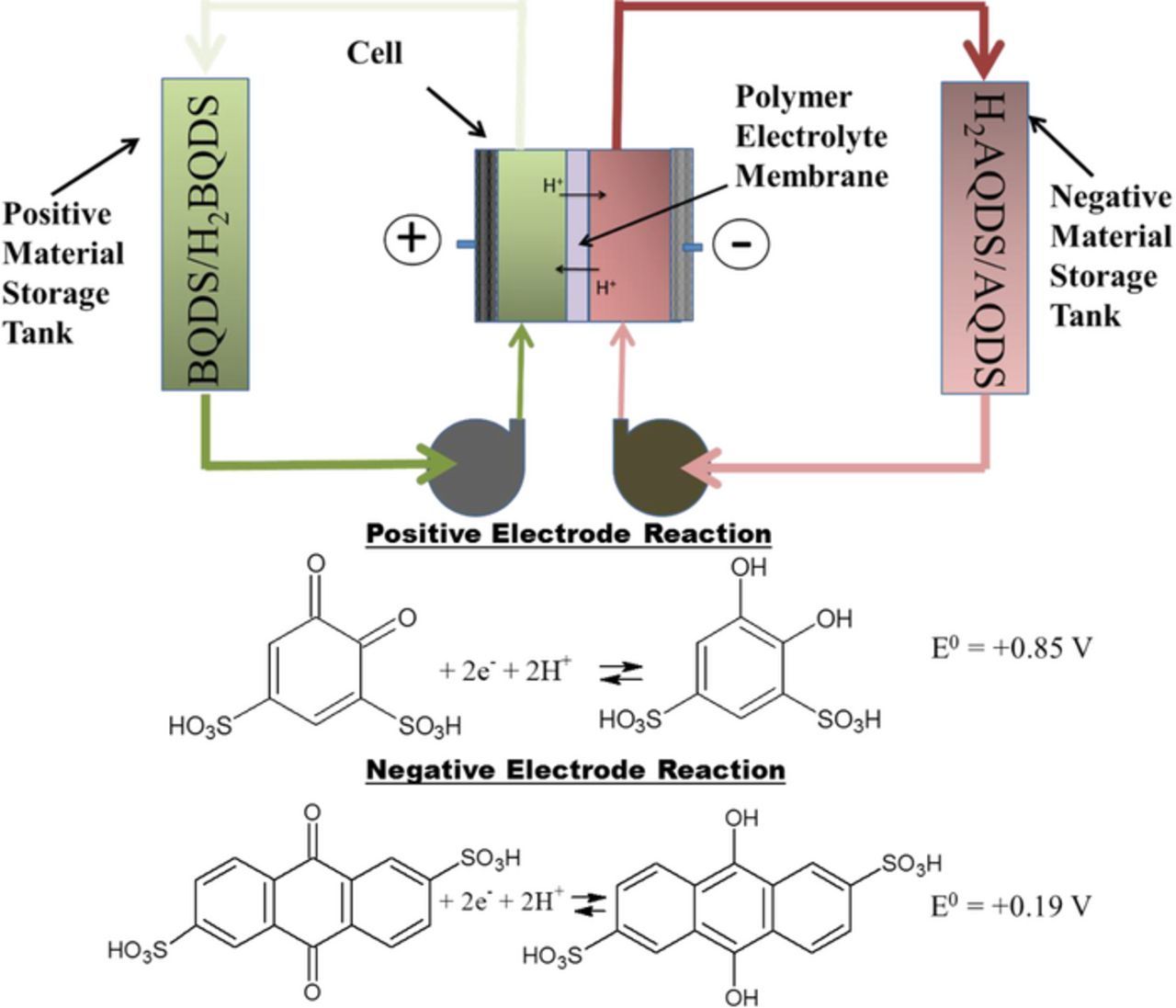
In 2013, we described an organic redox flow battery – also known as ORBAT – that uses water-soluble organic redox couples as a safe, scalable, and efficient energy storage system with the potential to meet the United States Department of Energy (DoE) cost target of $100/kWh for large-scale energy storage.4 In such a battery, aqueous solutions of two different water-soluble organic redox couples – quinones and anthraquinones or their derivatives - were circulated past carbon electrodes in an electrochemical cell. In our current system, the positive electrode is supplied with a solution of 4,5-dihydroxybenzene-1,3-disulfonic acid (BQDS) and the negative electrode uses a solution of anthraquinone-2,6-disulfonic acid (AQDS). The positive and negative electrode compartments are separated by a proton-conducting polymer electrolyte membrane (Figure 1). During charge and discharge, the redox couples undergo rapid proton-coupled electron transfer to store or release electrical energy. ORBAT does not rely on any heavy metals such as vanadium, chromium or zinc, and also avoids the use of volatile and flammable organic solvents such as those used in lithium-ion batteries. Additionally, the redox reactions do not require expensive precious metal catalysts. With the prospect of meeting the performance requirements and being inexpensive and safe, this battery is an attractive candidate for grid-scale energy storage.
Figure 1. Schematic of the operating principle of Organic Redox Flow Battery (ORBAT) using aqueous solutions of 4,5-dihydroxy-1,3-disulfonic acid (BQDS) on the positive side and anthraquinone-2,6-disulfonic acid (AQDS) on the negative side. Potential values are as reported in Reference 5.
Redox couples based on the aqueous electrochemistry of quinones undergo fast proton-coupled electron transfer and also provide a large charge capacity. We have measured the heterogeneous rate constants for the charge-transfer processes to be in the range of 10−3 to 10−4 cm s−1.5 The specific capacity for these molecules can range from 200 to 500 Ah/kg. Thus, such redox couples are highly suited for use in ORBAT. In addition, a variety of the molecular structures are possible with the quinones, allowing the tuning of their properties; substituent groups can be used to modify solubility in water, standard reduction potential, and the kinetics and mechanism of the redox reactions.6 The quinones are expected to cost about $10–15/kg or $20–30/kWh, leaving ample scope for achieving the cost target for the entire battery system of $100/kWh as set by US DoE, provided high power densities and voltage efficiencies can be achieved.7
While the oxidation-reduction properties of quinones and similar compounds have been known to electrochemists for many years, very few of these redox couples pairs have been exploited in rechargeable batteries.8–10 In 2014, we demonstrated a proof-of-concept for this type of battery design.5,11 Specifically, we demonstrated the feasibility of operating an aqueous redox flow cell with reversible water-soluble organic redox couples at both electrodes that could be charged and discharged multiple times at high faradaic efficiency without any sign of degradation. Until that time none of these compounds had been considered as an inexpensive and safe solution for large-scale energy storage applications.
However, in 2009, Xu et al. had studied the use of BQDS at the positive electrode and the lead/lead sulfate couple for the negative electrode.12 Aziz et al. has reported on a flow cell using aqueous solutions of anthraquinone disulfonic acid at the negative electrode and a conventional bromine/tribromide couple at the positive electrode.13–14 More recently, Aziz et al. have also reported a redox flow battery with an anthraquinone-based couple on the negative electrode with the ferro/ferricyanide couple at the positive electrode in alkaline media.15 Additionally, Aspuru-Guzik et al. published computational studies to identify quinone compounds that would be useful in an all-organic redox flow battery.16 While some of these molecules are predicted to show high positive electrode potentials, their chemical stability, solubility in aqueous media, and the propensity of the non-ionized forms to crossover through the cation exchange membrane need to be considered. Non-aqueous solutions of organic redox substances have been considered by the groups of Rasmussen, Brushett et al., and Abruña et al.17–19 Researchers at the Pacific Northwest National Laboratory have investigated the redox behavior of the stable free radical, TEMPO, in non-aqueous redox flow batteries.20–22
In our previous studies, we demonstrated the operation of ORBAT flow cells with 4,5-dihydroxybenzene-1,3-disulfonic acid at the positive electrode and anthraquinone-2,6-disulfonic acid at the negative electrode (Figure 1).5 Our analysis of the cell's performance showed that facile mass transport of reactants and products, high reactant/product solubility, and an open electrode structure with ample utilizable surface area was critical to achieve high current densities with these types of redox couples. In the proof-of-concept demonstration, we used a membrane-electrode assembly configuration similar to that used in the direct methanol fuel cell, without precious metal catalysts. As the cell configuration had not been optimized, we achieved only a modest value of power density of 0.025 W/cm2. Yet the demonstration of rechargeability and cycling of an ORBAT cell using solely aqueous solutions of quinone derivatives on both sides of the cell opened a new door to realizing an inexpensive, safe, and robust electrochemical system for large-scale energy storage.
In the present study, we report a ten-fold increase in the performance of the ORBAT cell. As a result, current densities of 200 mA/cm2 and a Coulombic efficiency of 100% have been achieved. However, we need to at least double the power density values to approach the performance level for some commercial vanadium-redox flow batteries.23–25 We also provide new understanding of the redox reactions of the quinones in acidic aqueous solutions and the role of electrode structure and flow field designs in determining the power density and efficiency of the cell. With such understanding, we project that further improvements in performance can be achieved.
Experimental
Basic electrochemical characterization
All experiments for the electrochemical characterization of the individual redox molecules were conducted in a standard three-electrode cell consisting of a rotating glassy-carbon disk working electrode, a platinum-wire counter electrode, and a mercury/mercuric sulfate reference electrode (E° = +0.65 V). The quinones, in either the fully-reduced or fully-oxidized form, were dissolved in 1 M sulfuric acid to a concentration of 1 mM. The solutions were de-aerated and kept under a blanket of argon gas throughout all the experiments. Linear-sweep voltammetry experiments were conducted at a scan rate of 5 mV s−1 over a range of rotation rates from 500 rpm to 3000 rpm, using a Versastat 300 potentiostat and rotating electrode equipment from Pine Instruments.
Charge/discharge cycling of full ORBAT cells
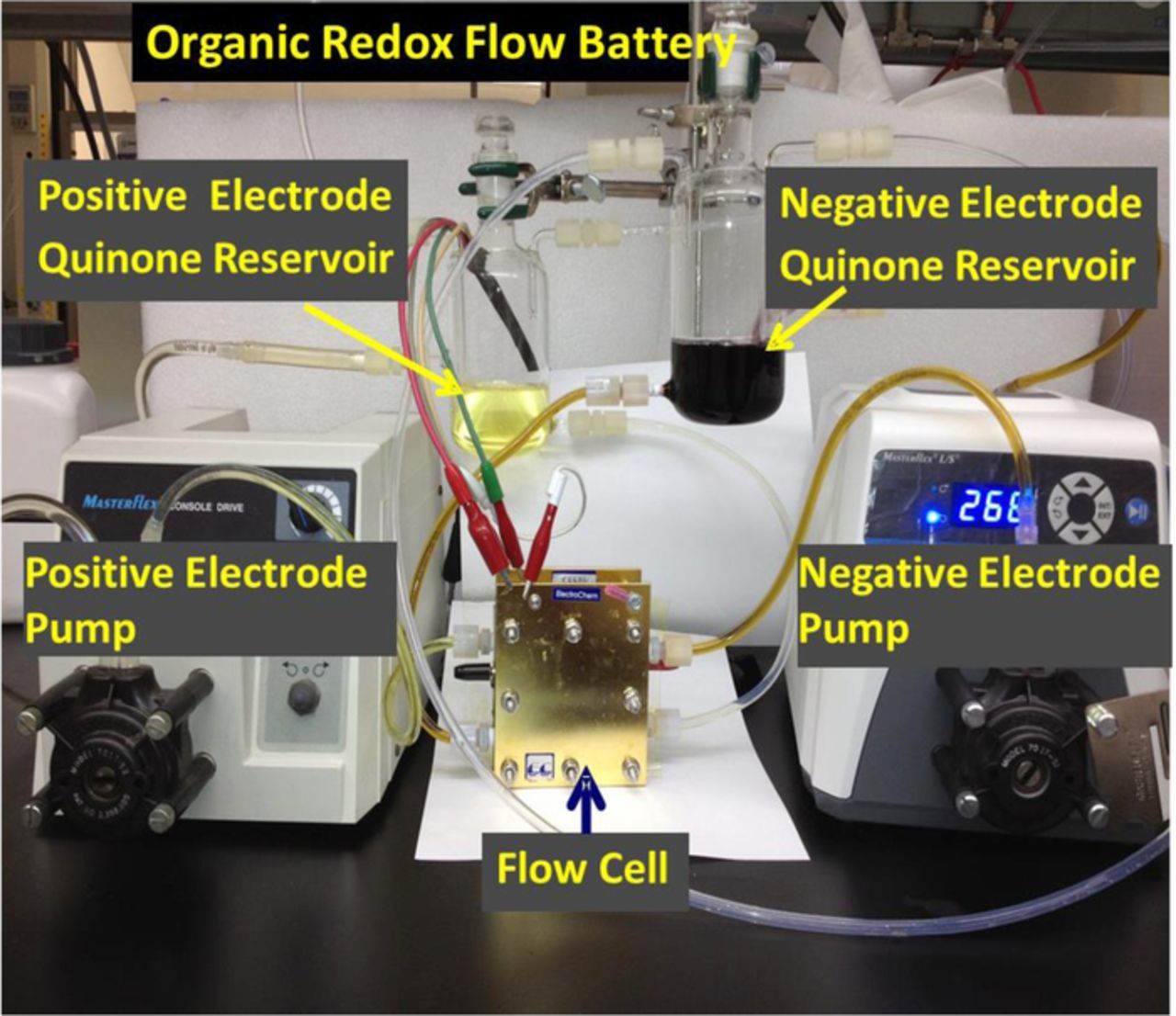
To build a flow cell, we adapted fuel cell test hardware (Electrochem Inc.) with an electrode active area of 25 cm2 and densified graphite flow field plates (Figure 2). The reactants were circulated using centrifugal pumps (March Pump Model #: BC-2CP-MD 12 V DC) at flow rates in the range of 0.5–1.0 liter min−1. Two flow-field designs that incorporated "flow-through" and "flow-by" features were tested. The "flow-through" configuration refers to a fluid path that includes flow that is perpendicular and parallel to the plane of the electrode; thus, the entire volume of the electrode has the opportunity to encounter the flow of solution. In a "flow-by" design, the fluid flow is largely parallel to the plane of the electrode. The relative utilization of the area of the electrodes in these two configurations is dependent on the flow rate and the pressure drop between the inlet and the outlet. We prepared the membrane electrode assemblies (MEAs) needed for the cell using procedures that were developed in-house, similar to those used in direct methanol fuel cells.26 Specifically, two sheets of carbon paper (Toray 030-non-teflonized, each with an area of 25 cm2) were coated with an ink made from 0.1 g of carbon black (Vulcan XC-72) and 0.3 g of Nafion with 0.5 ml of water and 0.5 ml of isopropanol. The equivalent weight of the Nafion ionomer was 1100 g/mole of protons. The coated electrodes were then hot pressed on to a Nafion 117 or 212 membrane to form the MEA. In some of the experiments, graphite felt (SIGRACELL GFD 4.6 from SGL Group) was used as electrodes, either as-received or after surface modification with Nafion and carbon black. This type of felt is widely used in vanadium-redox flow batteries.27 To increase their wettability, the felts were immersed in a dilute solution of Nafion and then dried at 140°C for an hour. The Nafion uptake was 10% of the weight of the felt. The felts were then boiled in water for 2 hours, to remove any excess or unbound Nafion and to hydrate the Nafion that was retained on the felt. In another modification, the graphite felt was coated with varying amounts of the carbon black.
Figure 2. Photograph of the test set-up for the flow cell studies.
All full cell experiments were carried out with solutions of redox couples dissolved in 1 M sulfuric acid at 23°C. Two glass containers served as reservoirs for the solutions of the redox couples (Figure 2). An argon flow was maintained at all times above these solutions to avoid reaction of the reduced form of the redox couples with oxygen. We have found that the exclusion of oxygen is critical to maintaining stable cell capacity. The current-voltage characteristics of the cells were measured at various states of charge. Charge/discharge studies were carried out under constant current conditions either using a potentiostat (Versastat 300) or a battery cycler (Maccor 4200). Typical currents ranged from 0.5 A to 5 A for an electrode area of 25 cm2.
Double-layer capacitance and electrochemically active surface area measurements
We used double-layer capacitance measurements to determine the electrochemically-active area of the electrodes. Electrochemical Impedance Spectroscopy (EIS) measurements were carried out over the frequency range of 10 mHz to 10 kHz with a sinusoidal excitation of amplitude ±2 mV peak-to-peak (Versastat 300 potentiostat). For these measurements, we circulated solutions containing solely 1 M sulfuric acid past both electrodes. Solutions were also carefully de-aerated to avoid oxygen. In the absence of faradaic reactions, the electrode/electrolyte interface is considered "ideally polarizable". Under these conditions, the imaginary component of the impedance, Zimag is related to the series equivalent capacitive reactance by the following equation:
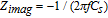
Where f is the excitation frequency and Cs is the value of the series-equivalent capacitance. The series-equivalent capacitance is also the double-layer capacitance because the interface in this experiment is ideally polarizable. Thus, the plot of Zimag vs. 1/(2πf) is expected to yield a line with a slope of −(1/Cs). The surface area is then calculated by assuming a specific capacitance of 20 microfarad /cm2 for a carbon surface and a geometric area of 25 cm2.
The ohmic resistance of the cells was determined from the real component of the complex impedance measured at 10 kHz. This value of ohmic resistance was used wherever the reported cell voltage was corrected for the voltage drop across the ohmic resistance of the electrodes, electrolyte and membrane. Such a correction allowed us to compare the mass transport and other kinetic limitations, ignoring variations in ohmic resistance caused by changes to the contact resistance resulting from changes to the flow field and compression of the electrodes. The correction was determined by multiplying the current with the ohmic resistance value, and subtracting this value from the cell voltage during charge or adding to the cell voltage value during discharge.
Nuclear magnetic resonance (NMR) studies
Samples withdrawn from cells were analyzed by proton-NMR to determine the transformations of the molecular structure of the quinone compounds. Typically, 100 μL of electrolyte sample was diluted with 0.5 mL of deuterated water (D2O) and 1H NMR spectra were obtained on Varian 400 MHz or 500 MHz NMR instruments with 128 scans. The chemical shifts were determined relative to the water signal (4.79 ppm).
Results and Discussion
Solubility of the acid form and sodium salt of the quinones
Our previous paper emphasized that high values of solubility of the redox couples is necessary for achieving high current densities and high efficiencies in ORBAT.5 We had used the sodium sulfonate salts of BQDS and AQDS (Sigma-Aldrich) "as received" in all our previous experiments. These salts exhibited solubility values ranging from 0.3 to 1.0 M in 1 M sulfuric acid at 23°C. When the sodium sulfonate salts of the quinones were transformed to the free acid form (sulfonic acid) by passing the solution through an ion-exchange column (Amberlyst 15(H) ion exchange resin), the solution concentrations of AQDS and BQDS could be increased to as high as 1.5 M and 4 M, respectively. For fully-dissociated electrolytes resulting in solvated ions in solution, solubility is governed by the difference between the crystal lattice energy and the solvation energy.28 The higher solubility observed for the acid form compared to the sodium salt is consistent with the solvation energy of a sodium ion being 162.7 kcal/mole less than that of a hydronium ion.29
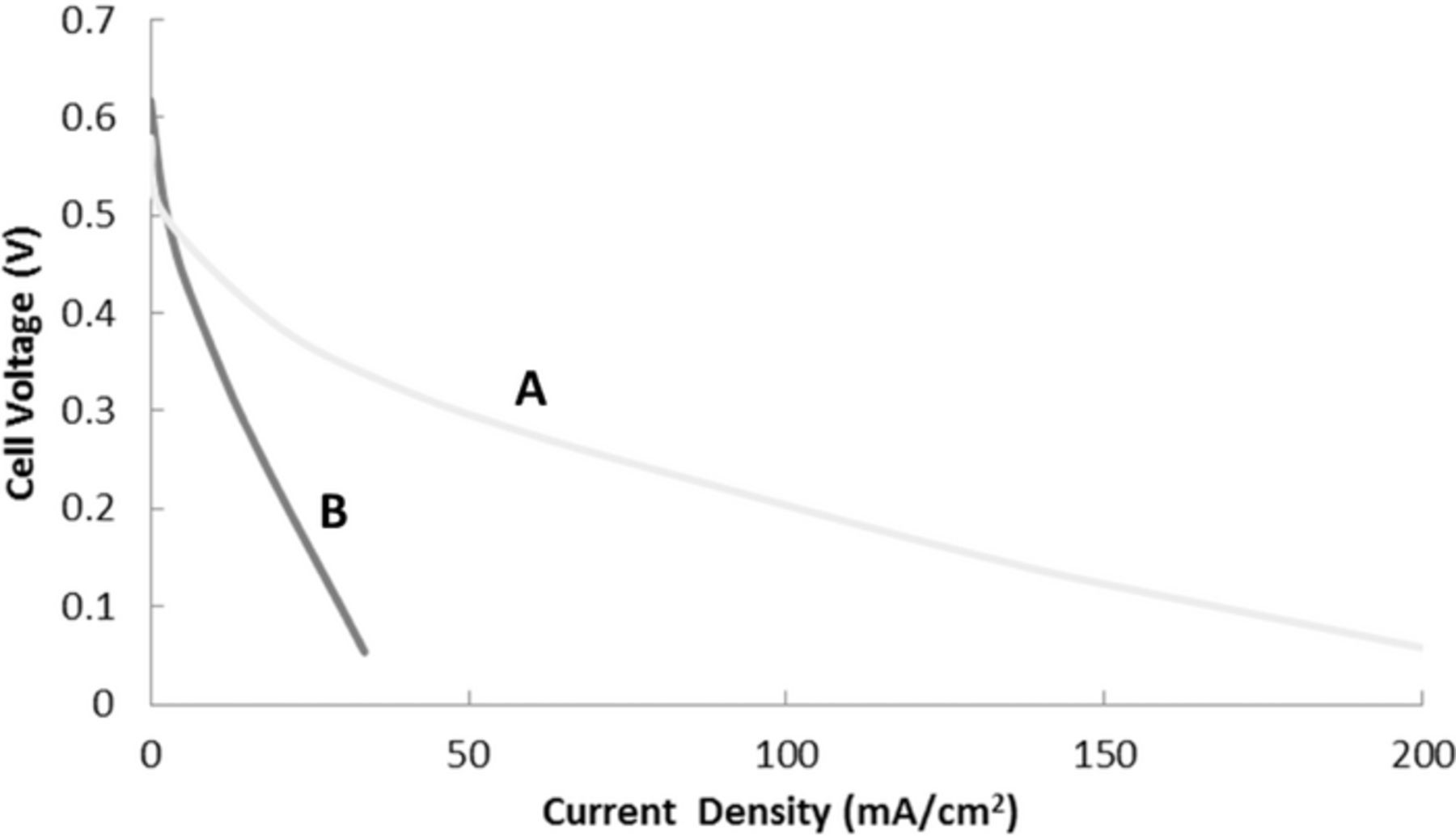
We observed a five-fold increase in current density with the free sulfonic acid form over the sodium salt form (Figure 3); this increase in performance can only be attributed to the increase in concentration of the reactants and improved ionic conductivity of the membrane. The higher solubility of the acid form avoided the abrupt drop in cell voltage resulting from concentration polarization caused by inadequate mass transport, especially at high current densities and low states-of-charge. The resistance of the cell at 10 kHz with 1 M sulfuric acid was 0.30 Ohm cm2, and the addition of 0.5 M solution of the sodium salt of AQDS raised this value of cell resistance to 0.58 Ohm cm2.Thus, the absence of sodium ions in the electrolyte increased the ionic conductivity of the Nafion membrane, as protons have a higher mobility in the membrane compared to the sodium ions.30,31
Figure 3. Current-voltage curves for the flow cell: 1 M of BQDS/AQDS in acid form (labeled A) and 0.2 M of BQDS/AQDS in sodium salt form (labeled B). Both cells employed Toray paper electrodes and a "flow-by" flow field design.
Charge/discharge cycling studies and transformation of 4,5-dihydroxybenzene-1,3-disulfonic acid
Solutions of 1 M AQDS and 2 M BQDS (both in the acid form) were circulated past the electrodes of the flow cell. In a typical experiment, 600 ml of the AQDS solution and 100 ml of the BQDS solution were used to give the mole ratio of 3:1 for AQDS to BQDS. We tried to use as high a concentration as possible to improve mass transport. However, since the solubility values for BQDS are substantially higher than that of AQDS even in the acid form, we had to use different concentrations for the positive and negative electrolyte solutions. The cell was charged and discharged multiple times to determine:
- (a)the chemical/electrochemical transformations that the redox couples undergo during charge and discharge (as determined by proton-NMR and linear sweep voltammetry), and
- (b)the propensity of the electroactive organic molecules to diffuse through the proton-conducting membrane, referred to hereafter as "crossover".
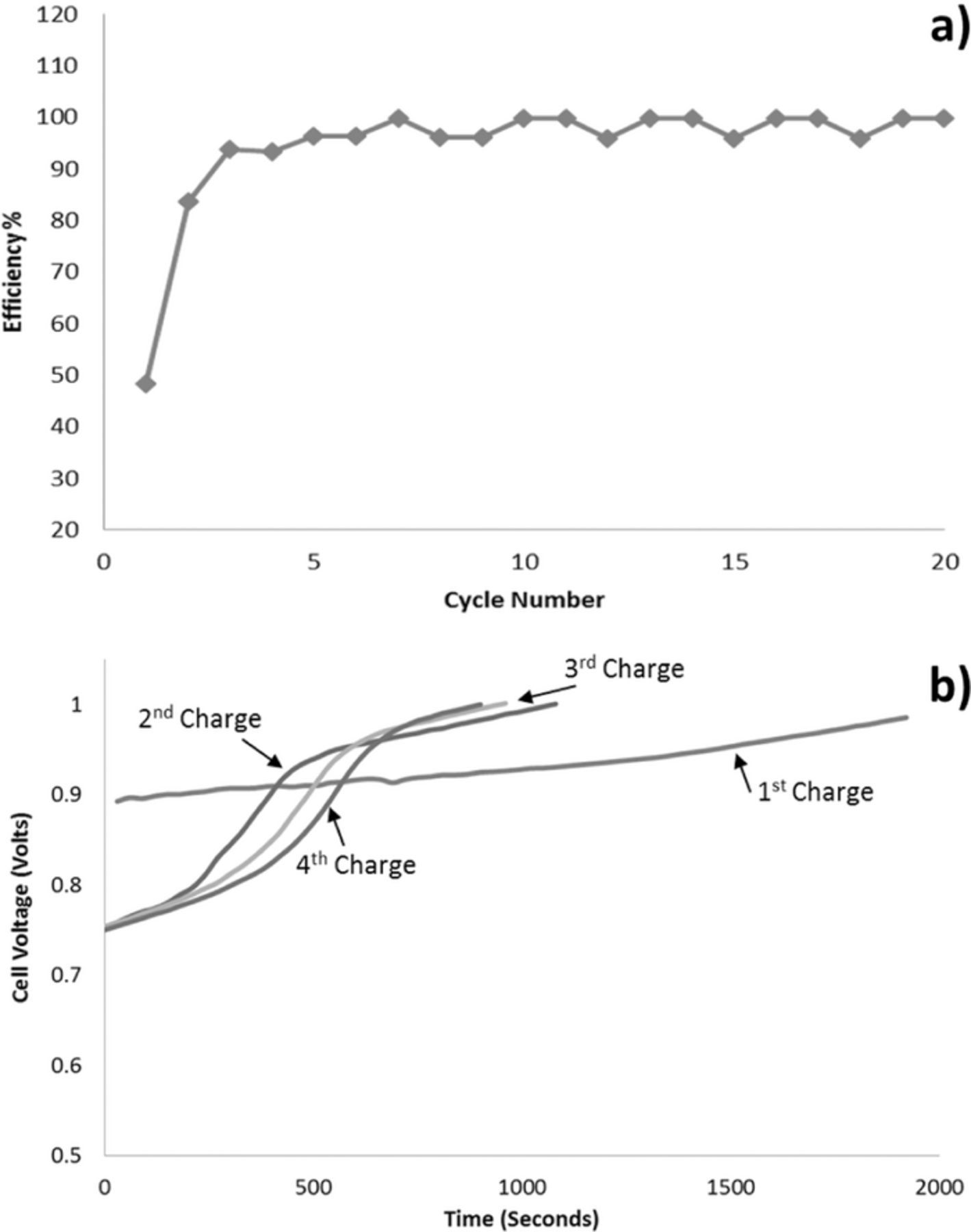
We found that stable capacity could be reached after about five cycles of charge and discharge (Figure 4). The charge acceptance in the first cycle was about three times that of the discharge capacity output following this charge. Therefore, the coulombic efficiency in the first cycle was about 33%. However, in subsequent cycles the coulombic efficiency gradually converged to 100% (Figure 4a). In the initial cycles, the charging curves showed a single voltage plateau that developed into two voltage plateaus (Figure 4b), suggesting the formation of at least two types of redox active species that underwent transformations. The initial value of the charging voltage of the cell before the transformations was 0.9 V (Figure 4b), and this reduced to 0.7 V by the end of 100 cycles, suggesting that chemical transformations of the redox active species had occurred during the cycling.
Figure 4. 25 cm2 redox flow cell, 0.2 M BQDS and AQDS, charged and discharged at 2A (80 mA/cm2) with graphite felt electrodes. a) Coulombic efficiency for the first 20 cycles when cycled between 1 V and 0 V (100% depth-of-discharge); b) Charge curves over the first four cycles. Corresponding discharge curves are included in the supplementary material as Figure S3.
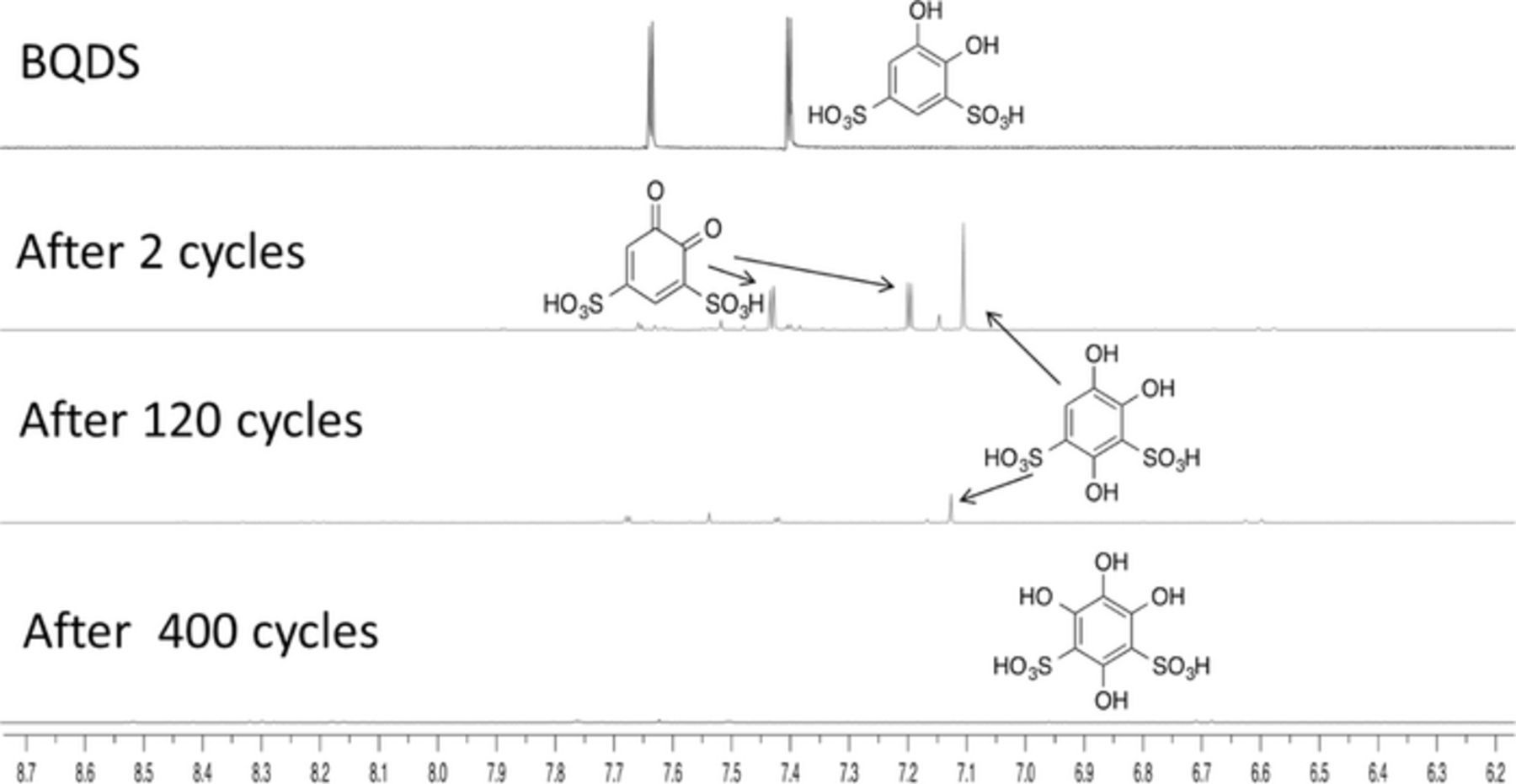
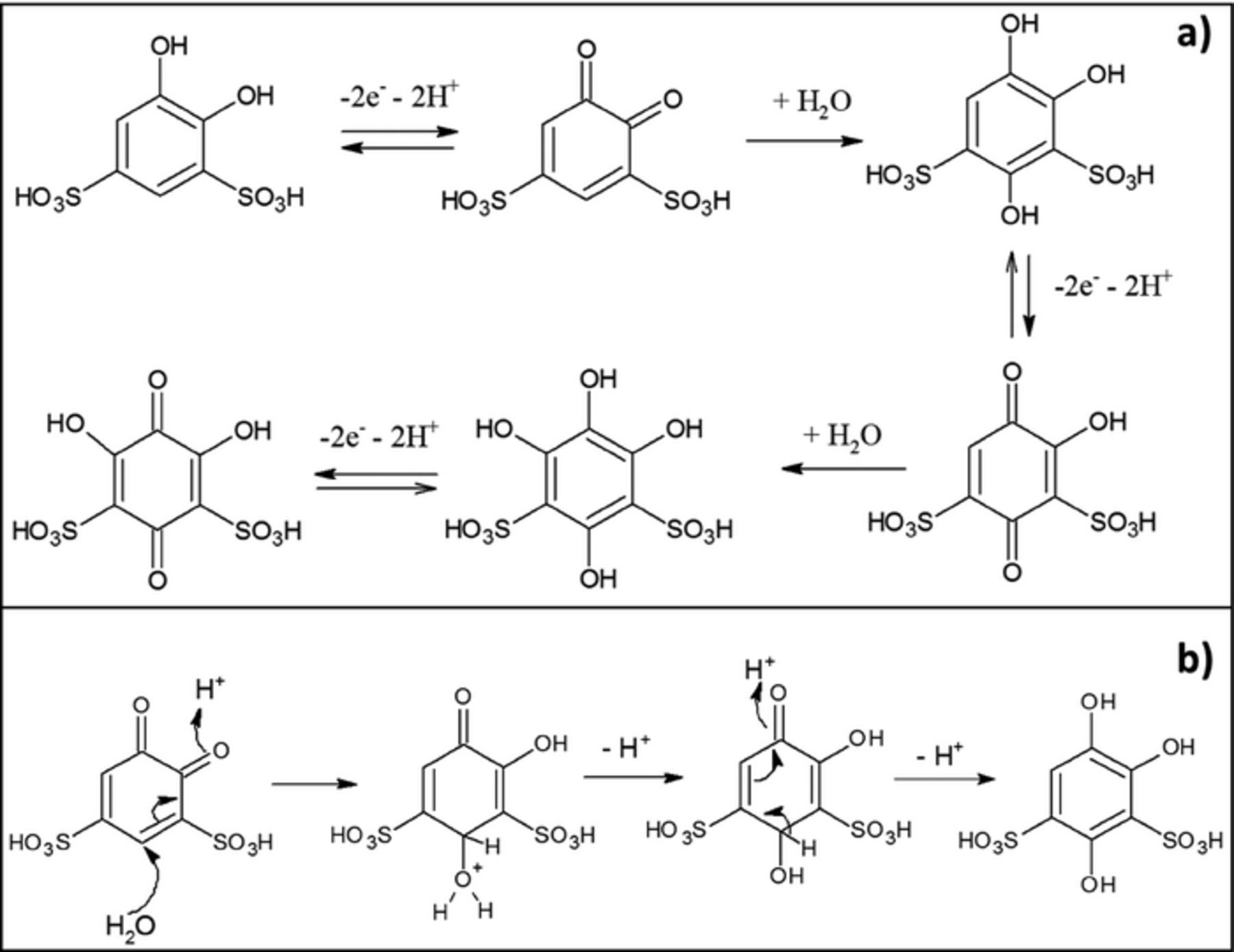
While the analysis of the negative electrode solution by proton NMR indicated no change of molecular composition of AQDS upon cycling, significant changes were observed in the solution at the positive electrode. Samples from the positive side of the cell drawn at different stages of the extended cycling at 100 mA/cm2 were analyzed by NMR (Figure 5). We determined that BQDS underwent a slow chemical transformation in addition to the expected electro-oxidation and electro-reduction during charge and discharge. The two doublets at 7.34 ppm (J = 2.2 Hz, 1H) and 7.10 ppm (J = 2.2 Hz, 1H) in the proton NMR at the start of the cycling corresponded to 4,5-dihydroxybenzene-1,3-disulfonic acid (BQDS), with protons on the aromatic ring present in two distinct environments. After the first charge, the 1H NMR spectrum showed a new "aromatic" proton signal at 7.14 ppm (singlet) that was attributed to 1,2,4-trihydroxybenzene-3,5-sulfonic acid. After extended cycling (120 cycles), the ratio of 1,2,4-trihydroxybenzene-3,5-sulfonic acid to BQDS had increased. After 400 cycles all the "aromatic" proton signals of BQDS and other reaction products disappeared, indicating the occurrence of yet another transformation that produced compounds with no peaks on the proton NMR spectrum. The lack of NMR-active "aromatic" protons on the aromatic ring suggested the formation of 1,2,4,6- tetrahydroxybenzene-3,5-sulfonic acid. Although the samples for NMR were obtained only after 120 cycles and 400 cycles, the electrochemical data indicated that changes were probably complete even in the earlier stages of cycling. The changes to the charge-discharge curves and the NMR spectra during cycling were consistent with the stepwise transformation of 4,5-dihydroxybenzene-1,3-disulfonic acid to 2,4,5-trihydroxybenzene-1,3-disulfonic acid and ultimately to 1,2,4,6-tetrahydroxybenzene-3,5-disulfonic acid (Figure 6). These transformations involve electrochemical oxidation of the hydroxyl group to the quinone, followed by the addition of water to form reduced products. The addition of nucleophiles such as water to the α,β-unsaturated carbonyl compounds in a 1,4-fashion is called the Michael reaction. In our study, the nucleophilic addition of water is accompanied by re-aromatization and exchange of the proton.32 The Michael reaction necessitated additional charge input for the re-oxidation of the product during charging.
Figure 5. 1H NMR at 400 or 500 MHz of BQDS samples taken after various numbers of cycles. Solutions were diluted with deutrated-water (D2O).
Figure 6. a) Schematic of the transformation of BQDS during charging; b) Schematic of the Michael reaction showing the nucleophilic addition of water followed by re-aromatization and proton exchange.
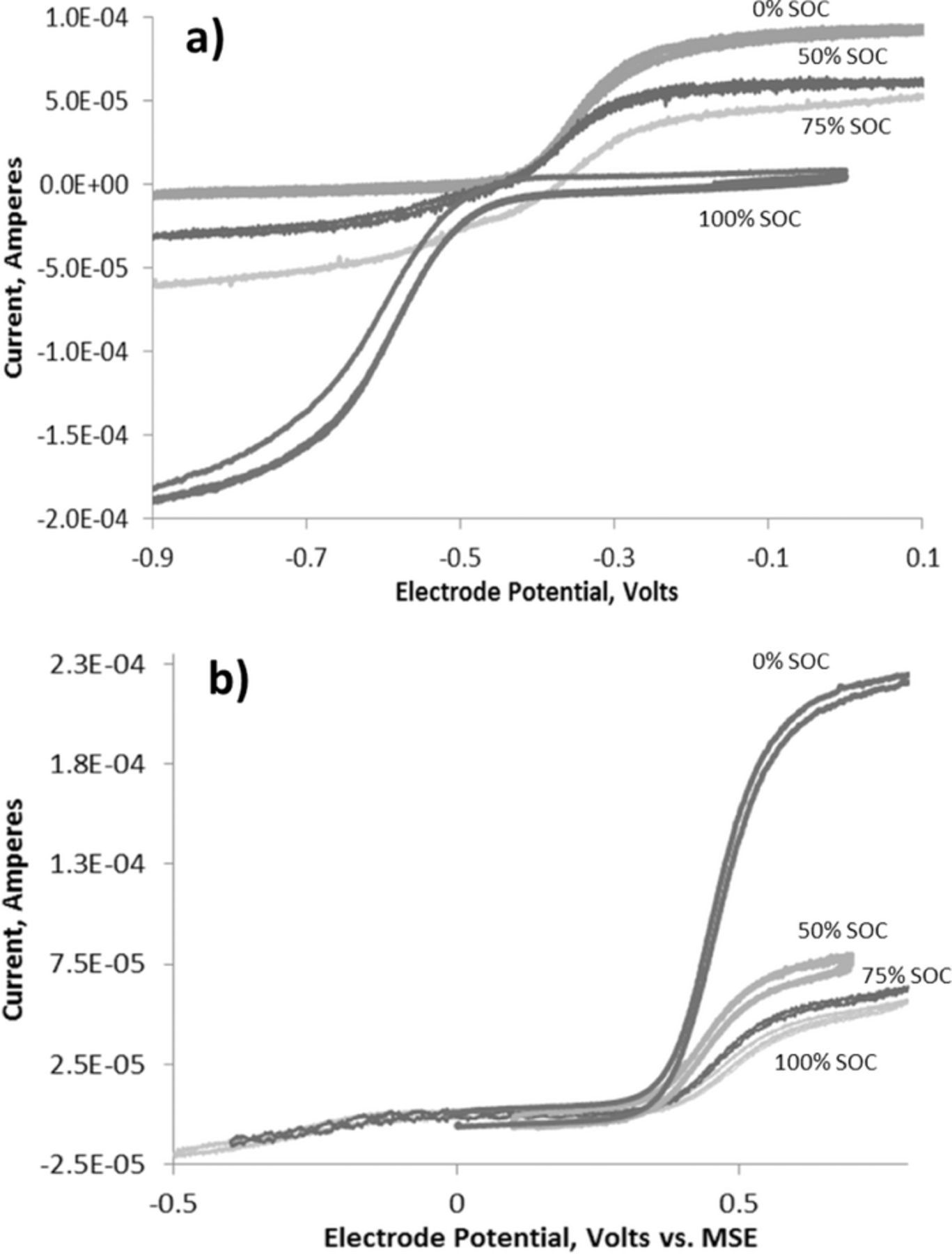
The limiting current at a rotating disk electrode during a linear sweep voltammetry experiment was used to analyze the progress of conversion of the materials from the discharged to the charged state. The value of the limiting current indicated the concentration of the reduced and oxidized species during charge and discharge. When AQDS was in its fully discharged form, there was a reduction current indicating presence of the oxidized form (Figure 7a). Similarly, when the solution on the negative side was in the fully-charged state there was no longer any reduction current − only an oxidation current − indicating the full conversion of the reactants.
Figure 7. Rotating disk electrode linear sweep voltammetry at glassy carbon disk (a) AQDS and (b) BQDS at various states of charge during cycling. The %SOC values refer to the amount of charge with reference to the theoretical discharge capacity based on BQDS. All scans were done at 5 mV/sec and at a rotation rate of 1500 rpm. Redox couple was at a concentration of 1 mM dissolved in 1 M sulfuric acid. We used a mercury/mercury sulfate reference electrode (E° = +0.650 V) and a platinum counter electrode.
However, the linear sweep voltammetry studies on BQDS indicated changes to the composition of the solution during the charging process. The persistent presence of the reduced state of the positive side material (Figure 7b) was consistent with the chemical transformations occurring during charging. The electrochemical studies confirmed that BQDS undergoes three redox transformations during charging that includes two Michael addition steps followed by re-aromatization with intermediates in the "reduced" state (Figure 6). Following these transformations, the 1,2,4,6-tetrahydroxybenzene-3,5-disulfonic acid became the positive electrode material that was repeatedly cycled against AQDS at the negative electrode.
The transformation of BQDS during the initial stages of cycling presented some challenges with the overall cell operation. First, at least three mole equivalents of AQDS were required compared to BQDS, in order to convert all the BQDS completely to its final form. After BQDS had reached its fully charged form, 66% of the AQDS remained unused during the subsequent cycles. Secondly, the products formed after the Michael reactions were expected to reduce the cell voltage, as additional hydroxyl substituents were added to BQDS. A reduction of about 150 mV in the charge voltage is noted after the first few cycles (Figure 4). This reduction is slightly lower than the 100 mV/ hydroxyl group expected from the study of substituent effects.6 We are in the process of synthesizing the transformed additive in the pure form and determining its standard reduction potential that is needed for understanding the energy efficiency values. Placing a fully-transformed BQDS (1,2,4,6-tetrahydroxybenzene -3,5-disulfonic acid) molecule in the cell at the start of cycling would avoid the need for excess AQDS. Synthesis of new molecules and electrochemical studies are currently underway to address these issues.
We confirmed from the NMR analysis of the solutions that even after 400 cycles that neither compound diffused through the Nafion membrane. These results were also consistent with the ex-situ studies− even after 2 months of holding solutions of 0.2 M AQDS or BQDS dissolved in 1 M sulfuric acid on one side of the membrane, and just 1 M sulfuric acid on the other side of the membrane, no crossover was detected. The absence of crossover confirmed the low permeability of the Nafion membrane to the anionic form of the redox molecules.
Effect of flow field modifications
Changes to the flow-field and electrode structure resulted in at least a three-fold increase in power density (Figure 8b). In these tests, an adequate amount of AQDS was used at the start of cycling tests to ensure complete conversion of BQDS. The flow field was modified from a flow-by configuration to an interdigitated design that added "flow-through" characteristics to the original flow field. We still had a small component (∼20%) of flow-by due to features that allowed some by-passing of the flow interdigitated structure (see Figure S1 in the Supplementary Material). With this improved design, the solutions were forced to move through the electrode structure, instead of simply flowing past the electrode. Several studies confirm the benefits of the flow-through design for redox flow batteries.23,33–36 By utilizing more of the surface area of the electrode and by improving mass transport to the electrode surface an increase in the cell performance was achieved. We note that the benefits of the flow-through design tended to increase with the flow rate. However, the flow-through design caused an increase in pressure drop for the flow of the solutions. The pressure drop was high when the carbon-coated Toray paper electrodes were used with a simple interdigitated flow field. With an open electrode structure, such as the SGL graphite felt, and with a combination of flow-through and flow-by designs, we could minimize the pressure drop and still maintain the high level of cell performance. We will continue pursuing the research on optimization of the flow-field design with open electrode structures for improving cell performance.
Figure 8. Polarization Curves (top figure) and power density curves (bottom figure) at 100% state-of-charge for A) 1 M AQDS/BQDS in 1 M Sulfuric acid, with a "flow-through" flow field and carbon-coated carbon felt electrodes and B) 1 M AQDS/BQDS in 1 M sulfuric acid, with a "flow-by" flow field and carbon-coated Toray paper electrodes.
Electrode design
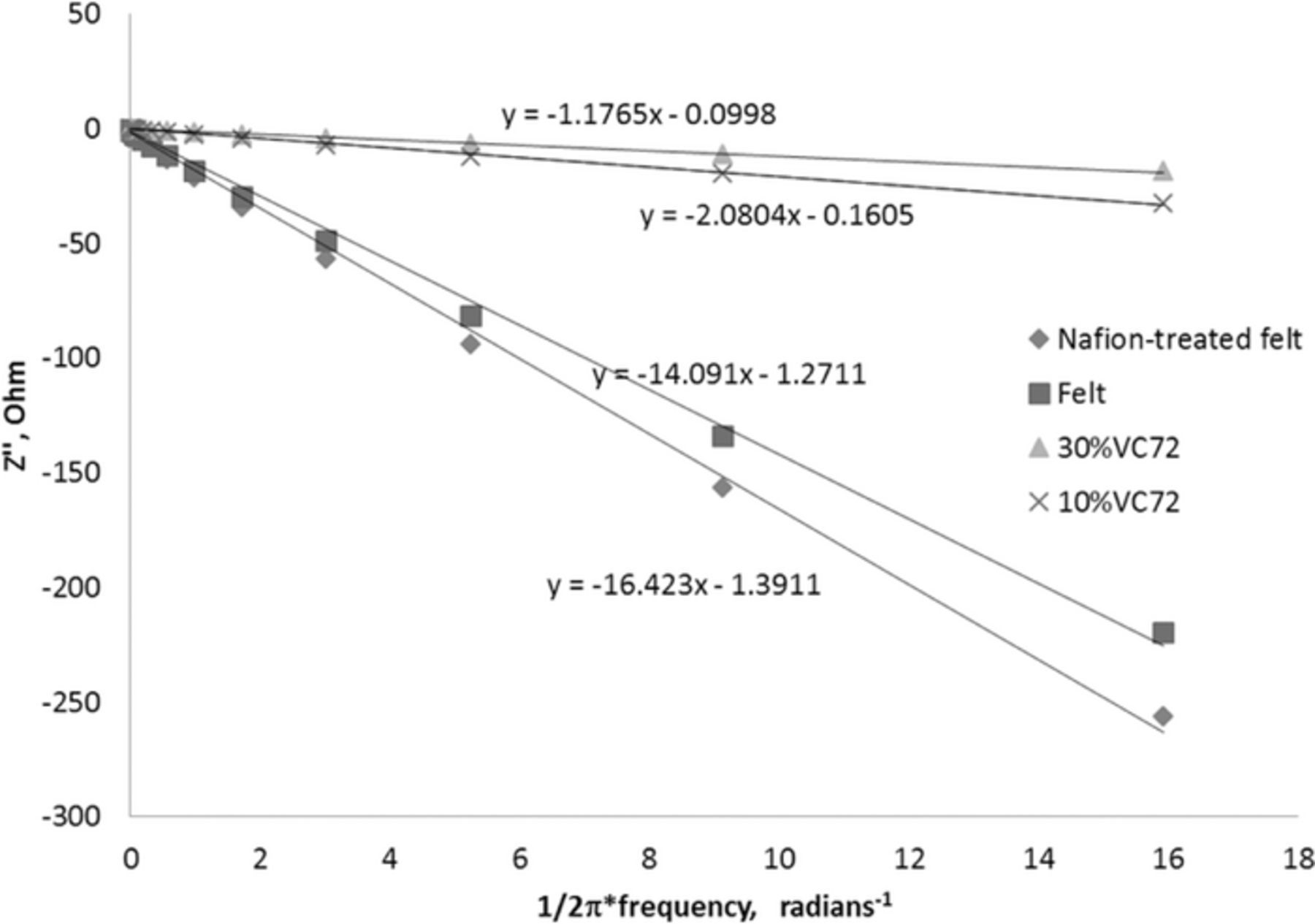
Efforts on improving the electrode design focused on increasing the accessible surface area of the electrodes. It is known that attaching high surface area carbon to electrode surfaces will improve performance in redox flow batteries.37 Double-layer capacitance measurements were used to determine the electrochemically active surface area. While the graphite felt treated with 2.5 wt% of Nafion appeared more wettable than the un-treated felt, both these samples had nearly the same double-layer capacitance (Figure 9, Table I). We expected that when the surface became more wettable more of the electrochemical active area would increase and consequently the double layer capacitance would increase. While Nafion increased the apparent wettability, it did not affect the accessible active area of the electrode to any significant degree. This observation suggests that the inaccessible area was in the micropores of the graphite fibers that were not affected by the addition of Nafion.
Figure 9. The imaginary component of the impedance plotted against the reciprocal of the angular frequency of excitation. The equations are for the line fits for the plotted data.
Table I. Double layer Capacitance and Electrochemically Active Surface Area of various types of SGL Carbon Felt (geometric area of 25 cm2) determined from the data in Figure 9.
| Eectrode Material (geometric are 25 cm2) | Capacitance (mF) | Electrochemically Active Surface Area (cm2) |
|---|---|---|
| Unmodified Graphite Felt | 2032.5 | 81.3 |
| 2.5% Nafion-Coated Graphite Felt | 2364.1 | 94.6 |
| 10% Carbon-Coated Graphite Felt | 16026 | 641.0 |
| 30% Carbon-Coated Graphite Felt | 28249 | 1129.9 |
The addition of Vulcan XC-72 carbon black into the graphite felt material greatly increased the double-layer capacitance (Table I). The incorporation of 30 weight % of the Vulcan XC-72 and 2.5% of Nafion in the graphite felt yielded the highest capacitance. Thus, the surface area of the electrode was substantially increased without any noticeable impact on the resistance to flow through the electrode structure. The graphite felt treated with 2.5 wt% of Nafion and 30% carbon black was employed in further optimization of ORBAT performance.
Power density
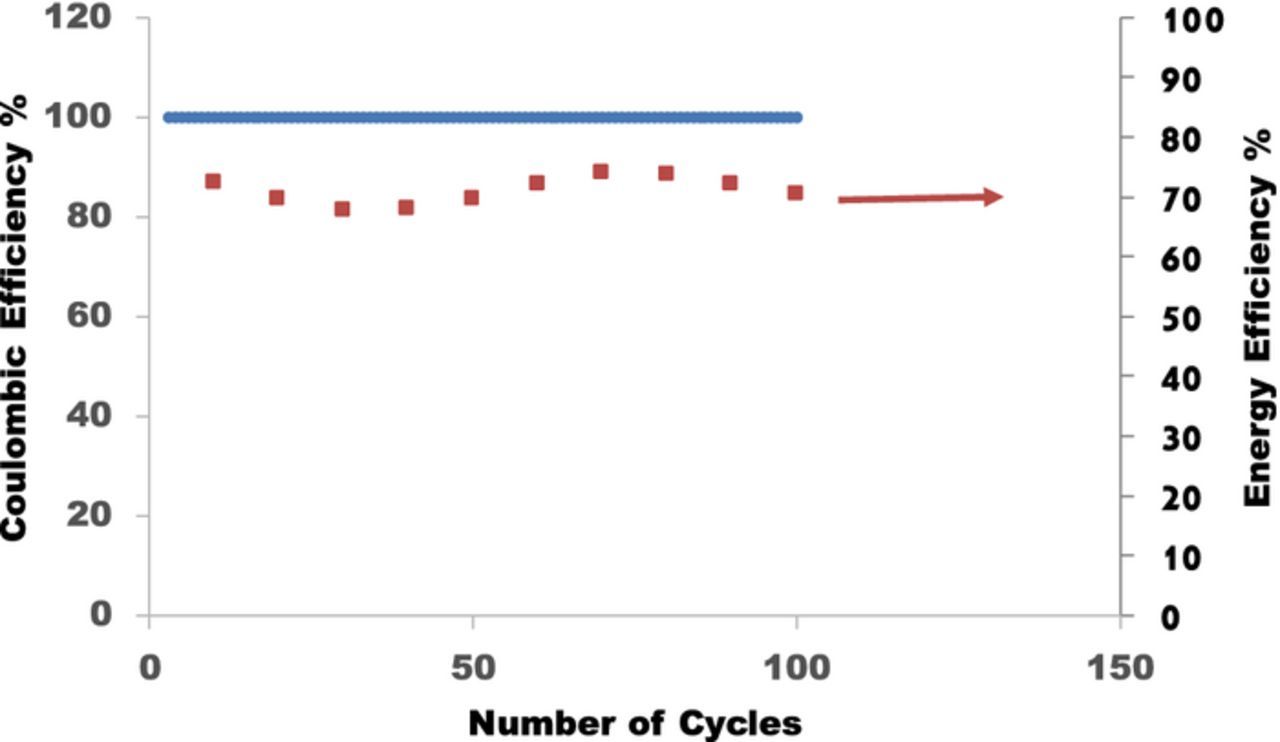
After making the improvements to the electrode structures and flow fields as described above, a power density as high as 100 mW/cm2 could be achieved with the high concentration of reactants in the acid form (Figure 8). Continuous cycling at a power density of 55 mW/cm2 at about 23°C could be sustained at a round-trip energy efficiency of 70% for at least a 100 cycles (Figure 10). We expect to achieve higher power density by replacing BQDS with a redox couple with a higher standard reduction potential that will avoid Michael reaction transformations. With further optimization of the flow field structure, reduction of the thickness of the electrodes, and small increases in operating temperature, we expect that a power density of 300 mW/cm2, similar to the standard operating power density of commercial vanadium redox flow batteries, can be achieved.24,25
Figure 10. Coulombic efficiency and energy efficiency over 100 cycles for a 25 cm2 flow cell with 1 M AQDS/BQDS with a modified interdigitated flow field and carbon-coated graphite felt electrodes (flow rate of 1 L/min). The cell was charged and discharged at 35% of its nominal starting capacity at 100 mA/cm2. Energy efficiency was calculated based on voltage-time curves that were corrected for the internal resistance.
Coulombic and energy efficiency
Maintaining 100% coulombic efficiency at all depths of discharge is essential for large-scale energy storage applications. The charge-discharge efficiency of 100% was maintained for at least 100 cycles after the transformations in the first 4 to 5 cycles (Figure 10). Capacities as large as 5 Ah were realized from the cells. While the initial charge-discharge efficiency was low due to the afore-mentioned chemical transformations of BQDS, the charge-discharge efficiency stayed close to 100% once the transformations were complete. The value of 100% coulombic efficiency over the 100 cycles is consistent with the absence of crossover as confirmed by the electrochemical measurements, NMR data, and independent ex-situ studies.
The round-trip energy efficiency of ORBAT when charged and discharged at 100 mA/cm2 was about 70% over 100 cycles. In each cycle 35% of the total capacity was introduced during charge and the same quantity was withdrawn during discharge. The voltage cutoff limits were held at 1.0 V and 0.0 V during charge and discharge, although these were not reached during the 100 cycles of testing. The electrolyte path length in the graphite felt electrodes (thickness of 2.5–3.0 mm) contributed significantly to the ohmic resistance of the cell. This resistance was considerably less with Toray paper electrodes. Thus, the value of energy efficiency was determined from the area under the charge and discharge curves after correcting the cell voltage for losses from the ohmic resistance in the cell. The value of energy efficiency can be improved further by reducing the mass transport losses. Such optimization studies are currently underway.
Conclusions
We have demonstrated for the first time the feasibility of repeatedly cycling an aqueous redox flow cell with reversible water-soluble organic redox couples (ORBAT), a round-trip efficiency of 70% at 100 mA/cm2 at 100% coulombic efficiency. In the present study, the cells were successfully operated with concentrated solutions of 4,5-dihydroxybenzene-1,3-disulfonic acid (BQDS) at the positive electrode and anthraquinone-2,6-disulfonic acid (AQDS) at the negative electrode. During the initial cycles of charge and discharge, the BQDS was converted to 1,2,4,6- tetrahydroxybenzene-3,5-sulfonic acid via two steps of electrochemical oxidation and two steps of water addition through the Michael reaction. After this conversion, no further change in the composition of the positive electrode solution was observed. The 100% coulombic efficiency was demonstrated over 100 cycles. These cells could be cycled at 100 mA/cm2 at room temperature. These performance improvements have resulted from reduction of the voltage losses arising primarily from mass transport limitations. Using the free acid form of these organic redox couples in acidic media was crucial for achieving the necessary high concentrations that in turn resulted in the high current density and high energy efficiency. The flow-through configuration for the flow field improved the electrode utilization substantially. High-surface area carbon black can be used to modify the felt electrode to increase the electrochemically-active surface area of the electrodes. We have identified the various chemical transformations of BQDS during the charging process; this understanding has helped explain the evolution of the coulombic efficiency values during the initial five cycles and the need for three times more AQDS than BQDS for achieving the highest capacities. We were able to confirm that the crossover of the reactants through the membrane was not significant over at least 400 cycles.
We are pursuing further improvements to the performance of ORBAT by the optimization of the combination of the flow-field and high-surface area electrode structures, increasing the operating temperature to 50–60°C, and by preventing the Michael reaction on the positive electrode material. While we understand the techno-economic limitations of low voltage RFB systems, our study provides understanding and insight into the basic electrochemistry and transformations occurring in the operation of RFBs based on water-soluble organic redox couples. We expect that the results of our study can be usefully extended to other systems, while we continue to pursue the discovery of redox couples for higher voltage systems. We expect the performance improvements and new understanding reported here to hasten the development of ORBAT as an inexpensive and sustainable solution for large-scale energy storage.
Acknowledgment
We acknowledge the financial support for this research from the ARPA-E Open-FOA program (DE-AR0000337), the University of Southern California, and the Loker Hydrocarbon Research Institute.