Abstract
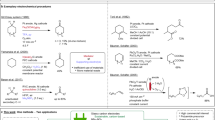
The electroreductive coupling of activated olefins and 1–3 dibromide reported in a previous paper leads to a cyclic product when the reaction is carried out in an undivided cell in NMP and in the presence of a sacrificial aluminum anode, but not in a divided cell A mechanistic study has been made to explain the discrepancy between the two methods. We report here that in the undivided cell and in the presence of anodically generated aluminum ions the electroreduction of activated olefins mainly lead to a dianion, while a radical anion is the main reactive species under the other experimental conditions.
Similar content being viewed by others
References and Notes
J.P. Petrovich, M.M. Baizer, and M.R. Ort, J. Electrochem. Soc., 116(1969)743.
V.J. Puglisi and A.J. Bard, J. Electrochem. Soc., 119(1972)829.
E. Lamy, L. Nadjo, and J.M. Saveant, J. Electroanal. Chem., 42(1973)189.
C. Degrand and H. Lund, Nouveau J. de Chimie, 1(1977)35.
H. Lund, M.A. Michel, and J. Simonet, Acta Chem. Scand. B., 28(1974)900.
S. Margel and M. Levy, Electroanal. Chem. Interf. Electrochem., 56(1974)259.
M.M. Baizer and J.L. Chruma, J. Org. Chem., 37(1972)1951.
S. Satoh, H. Suginome, and M. Tokuda, Tetrahedron Lett., 20(1981)1895.
S. Satoh, H. Suginome, and M. Tokuda, Bull. Chem. Soc. Jpn., 54(1981)3456.
S. Satoh, T. Taguchi, M. Itoh, and M. Tokuda, Bull. Chem. Soc. Jpn., 52(1979)951.
Y.W. Lu, J.Y. Nédélec, J.C. Folest, and J. Pénchon, J. Org. Chem., 55(1990)2503.
D. Griller and K.U. Ingold, Acc. Chem. Res., 13(1980)317.
M. Newcomb and A.G. Glenn, J. Am. Chem. Soc., 111(1989)275.
A.L.J. Beckwith and V.W. Bowry, J. Org. Chem., 54(1989)2681.
Unpblished.
We do not ignore that finding the ring-open product does not necessarily infer that the mechanssm involves the cyclopropylmethyl radical as the key intermediate (see Ref. 17). But assuming a non-radical mechanism with styrene would make difficult the understanding of the behaviour of the reagent mixture containing either 2 or 3 in the undivided cell when the reaction was carried out in the presence of aluminum ions.
M. Newcomb and D.P. Cumin, Acc. Chem. Res., 21(1988)206.
The underconsumpiion of electricity reflects the extent of polymerisation (see Ref. 2).
K.G. Bilyard, P.J. Garatt, R. Hunter, and E. Lete, J. Org. Chem., 47(1982)473.
D. Wilkemng, B.P. Mundy, and K.B. Lipkowitz, J. Org. Chem., 50(1985)5727.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lachaise, I., Lu, Y.W., Nédélec, J.Y. et al. Intermediate and reaction mechanism of the electroreductive coupling of activated olefins and alkyl dihalides using the sacrificial anode process. Res Chem Intermed 15, 253–260 (1991). https://doi.org/10.1163/156856791X00363
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856791X00363