Abstract
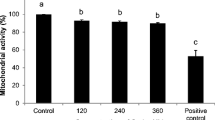
We investigated the ability of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), hydroxyl radical (·OH), and hypochlorous acid (HOCl), to overcome the defensive capacity of cumulus cells and elucidate the mechanism through which ROS differentially deteriorate oocyte quality. Metaphase II mouse oocytes with (n = 1634) and without cumulus cells (n = 1633) were treated with increasing concentration of ROS, and the deterioration in oocyte quality was assessed by the changes in the microtubule morphology and chromosomal alignment. Oocyte and cumulus cell viability and cumulus cell number were assessed by indirect immunofluorescence, staining of gap junction protein, and trypan blue staining. The treated oocytes showed decreased quality as a function of increasing concentrations of ROS when compared to controls. Cumulus cells show protection against H2O2 and ·OH insult at lower concentrations, but this protection was lost at higher concentrations (>50 μmol/L). At higher H2O2 concentrations, treatment dramatically influenced the cumulus cell number and viability with resulting reduction in the antioxidant capacity making the oocyte more susceptible to oxidative damage. However, cumulus cells offered no significant protection against HOCl at any concentration used. In all circumstances in which cumulus cells did not offer protection to the oocyte, both cumulus cell number and viability were decreased. Therefore, the deterioration in oocyte quality may be caused by one or more of the following: a decrease in the antioxidant machinery by the loss of cumulus cells, the lack of scavengers for specific ROS, and/or the ability of the ROS to overcome these defenses.
Similar content being viewed by others
References
Motta PM, Nottola SA, Pereda J, Croxatto HB, Familiari G. Ultra-structure of human cumulus oophorus:a transmission electron microscopic study on oviductal oocytes and fertilized eggs. Hum Reprod. 1995; 10(9):2361–2367.
Huang Z, Wells D. The human oocyte and cumulus cells relationship:new insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010; 16(10):715–725.
D’Alessandris C, Canipari R, Di Giacomo M, et al. Control of mouse cumulus cell-oocyte complex integrity before and after ovulation:plasminogen activator synthesis and matrix degradation. Endocrinology. 2001; 142(7):3033–3040.
Wang Q, Chi MM, Schedl T, Moley KH. An intercellular pathway for glucose transport into mouse oocytes. Am J Physiol Endocrinol Metab. 2012; 302(12):E1511–E1518.
Hashimoto S, Saeki K, Nagao Y, Minami N, Yamada M, Utsumi K. Effects of cumulus cell density during in vitro maturation of the developmental competence of bovine oocytes. Theriogenol-ogy. 1998; 49(8):1451–1463.
Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol. 2000; 226(2):167–179.
Goodenough DA, Simon AM, Paul DL. Gap junctional intercellular communication in the mouse ovarian follicle. Novartis Foundation Symp. 1999; 219:226–235; discussion 35–40.
Matzuk MM, Bums KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary:oocytes carry the conversation. Science. 2002; 296(5576):2178–2180.
Lee RK, Li SH, Lu CH, Ho HY, Chen YJ, Yeh HL Abnormally low expression of connexin 37 and connexin 43 in subcutaneously transplanted cryopreserved mouse ovarian tissue. J Assist Reprod Genet. 2008; 25(9–10):489–497.
Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life. 2001; 51(1):57–64.
Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Effect of lactate dehydrogenase activity and isoenzyme localization in bovine oocytes and utilization of oxidative substrates on in vitro maturation. Theriogenology. 1999; 51(3):541–550.
Goud PT, Goud AP, Joshi N, Puscheck E, Diamond MP, Abu-Soud HM. Dynamics of nitric oxide, altered follicular microenvir-onment, and oocyte quality in women with endometriosis. Fertil Steril. 2014; 102(1):151–159.
Goud AP, Goud PT, Diamond MP, Gonik B, Abu-Soud HM. Reactive oxygen species and oocyte aging:role of superoxide, hydrogen peroxide, and hypochlorous acid. Free Radic Biol Med. 2008; 44(7):1295–1304.
Rajani S, Chattopadhyay R, Goswami SK, Ghosh S, Sharma S, Chakravarty B. Assessment of oocyte quality in polycystic ovarian syndrome and endometriosis by spindle imaging and reactive oxygen species levels in follicular fluid and its relationship with IVF-ET outcome. J Hum Reprod Sci. 2012; 5(2):187–193.
Papaleo E, Ottolina J, Vigano P, et al. Deep pelvic endometriosis negatively affects ovarian reserve and the number of oocytes retrieved for in vitro fertilization. Acta Obstet Gynecol Scand. 2011; 90(8):878–884.
Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction:a review. Reprod Biol Endocrinol. 2012; 10:49.
Shaeib F, Banerjee J, Maitra D, Diamond MP, Abu-Soud HM. Impact of hydrogen peroxide-driven Fenton reaction on mouse oocyte quality. Free Radic Biol Med. 2013; 58:154–159.
Banerjee J, Maitra D, Diamond MP, Abu-Soud HM. Melatonin prevents hypochlorous acid-induced alterations in microtubule and chromosomal structure in metaphase-II mouse oocytes. J Pineal Res. 2012; 53(2):122–128.
Loschen G, Azzi A, Richter C, Flohe L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS lett. 1974; 42(1):68–72.
Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases:from molecular mechanisms to health implications. Antioxid Redox Signal. 2008; 10(7):1199–1234.
Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 2nd ed. Oxford:Clarendon Press; 1989:1–20
Agarwal A, Allamaneni SS. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online. 2004; 9(3):338–347.
Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS lett. 2000; 486(1):10–13.
Liu L, Trimarchi JR, Keefe DL. Involvement of mitochondria in oxidative stress-induced cell death in mouse zygotes. Biol Reprod. 2000; 62(6):1745–1753.
Liu L, Keefe DL. Cytoplasm mediates both development and oxidation-induced apoptotic cell death in mouse zygotes. Biol Reprod. 2000; 62(6):1828–1834.
Fujino Y, Ozaki K, Yamamasu S, et al. DNA fragmentation of oocytes in aged mice. Hum Reprod. 1996; 11(7):1480–1483.
Perez GI, Tilly JL. Cumulus cells are required for the increased apoptotic potential in oocytes of aged mice. Hum Reprod. 1997; 12(12):2781–2783.
Velez-Pardo C, Morales AT, Del Rio MJ, Olivera-Angel M. Endogenously generated hydrogen peroxide induces apoptosis via mitochondrial damage independent of NF-kappaB and p53 activation in bovine embryos. Theriogenology. 2007; 67(7):1285–1296.
Korotkova EI, Misini B, Dorozhko EV, Bukkel MV, Plotnikov EV, Linert W. Study of OH radicals in human serum blood of healthy individuals and those with pathological schizophrenia. Int J Mol Sci. 2011; 12(1):401–410.
Tahboub YR, Galijasevic S, Diamond MP, Abu-Soud HM. Thio-cyanate modulates the catalytic activity of mammalian peroxidases. J Biol Chem. 2005; 280(28):26129–26136.
Kettle AJ, Winterboum CC. Assays for the chlorination activity of myeloperoxidase. Methods Enzymol. 1994; 233:502–512.
Riley CF, Moen MH, Videm V. Inflammatory markers in endometriosis:reduced peritoneal neutrophil response in minimal endometriosis. Acta Obstet Gynecol Scand. 2007; 86(7):877–881.
Lamaita RM, Pontes A, Belo AV, et al. Evaluation of N-acetilglucosaminidase and myeloperoxidase activity in patients with endometriosis-related infertility undergoing intracytoplas-mic sperm injection. J Ofctet Gynaecol Res. 2012; 38(5):810–816.
Klebanoff SJ. Myeloperoxidase:friend and foe. J Leukocyte Biol. 2005; 77(5):598–625.
Larman MG, Minasi MG, Rienzi L, Gardner DK. Maintenance of the meiotic spindle during vitrification in human and mouse oocytes. Reprod Biomed Online. 2007; 15(6):692–700.
Rienzi L, Martinez F, Ubaldi F, et al. Polscope analysis of meiotic spindle changes in living metaphase II human oocytes during the freezing and thawing procedures. Human Reprod. 2004; 19(3):655–659.
Eroglu A, Toth TL, Toner M. Alterations of the cytoskeleton and polyploidy induced by cryopreservation of metaphase II mouse oocytes. Fertility Steril. 1998; 69(5):944–857.
Lindley EM, Jacobson JD, Corselli J, King A, Chan PJ. Cryopreservation of human cumulus cells for co-cultures and assessment of DNA damage after thawing using the comet assay. J Assist Reprod Genet. 2001; 18(10):534–538.
Ohashi Y, Kan Y, Watanabe T, Honda Y. Redox silencing of the Fenton reaction system by an alkylitaconic acid, ceripo-ric acid B produced by a selective lignin-degrading fungus, Ceriporiopsis subvermispora. Org Biomol Chem. 2007; 5(5):840–847.
Banerjee J, Shaeib F, Maitra D, et al. Peroxynitrite affects the cumulus cell defense of metaphase II mouse oocytes leading to disruption of the spindle structure in vitro. Fertil Steril. 2013; 100(2):578–584.
Choi WJ, Baneijee J, Falcone T, Bena J, Agarwal A, Sharma RK. Oxidative stress and tumor necrosis factor-alpha-induced alterations in metaphase II mouse oocyte spindle structure. Fertil Steril. 2007; 88(4 suppl):1220–1231.
Boiso I, Marti M, Santalo J, Ponsa M, Barri PN, Veiga A. A con-focal microscopy analysis of the spindle and chromosome configurations of human oocytes cryopreserved at the germinal vesicle and metaphase II stage. Hum Reprod. 2002; 17(7):1885–1891.
Sliskovic I, Abdulhamid I, Sharma M, Abu-Soud HM. Analysis of the mechanism by which tryptophan analogs inhibit human myeloperoxidase. Free Radic Biol Med. 2009; 47(7):1005–1013.
Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952; 195(1):133–140.
Wang L, Bassiri M, Najafi R, et al. Hypochlorous acid as a potential wound care agent:part I. Stabilized hypochlorous acid:a component of the inorganic armamentarium of innate immunity. J Burns Wounds. 2007; 6:e5.
Maitra D, Shaeib F, Abdulhamid I, et al. Myeloperoxidase acts as a source of free iron during steady-state catalysis by a feedback inhibitory pathway. Free Radic Biol Med. 2013; 63:90–88.
Prousek J. Fenton chemistry in biology and medicine. Pure Appl Chem. 2007; 79(12):2325–2338.
Crichton RR, Wilmet S, Legssyer R, Ward RJ. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J Inorg Biochem. 2002; 91(1):9–18.
Defrere S, Lousse JC, Gonzalez-Ramos R, Colette S, Donnez J, Van Langendonckt A. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol Hum Reprod. 2008; 14(7):377–385.
Yamaguchi K, Mandai M, Toyokuni S, et al. Contents of endome-triotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative sfress. Clin Cancer Res. 2008; 14(1):32–40.
Lizuka M, Igarashi M, Abe Y, Ibuki Y, Koyasu Y, Ikuma K. Chemical assay of iron in ovarian cysts:a new diagnostic method to evaluate endomefriotic cysts. Gynecol Obstet Invest. 1998; 46(1):58–60.
Spolarics Z, Wu JX. Role of glutathione and catalase in H202 detoxification in LPS-activated hepatic endothelial and Kupffer cells. Am J Physiol. 1997; 273(6 pt 1):G1304–G1311.
Dayer R, Fischer BB, Eggen RI, Lemaire SD. The peroxiredoxin and glutathione peroxidase families in Chlamydomonas reinhard-tii. Genetics. 2008; 179(1):41–57.
Goyer A, Haslekas C, Miginiac-Maslow M, et al. Isolation and characterization of a thioredoxin-dependent peroxidase from Chlamydomonas reinhardtii. Eur J Biochem. 2002; 269(1):272–282.
Sikka SC. Role of oxidative stress and antioxidants in androl-ogy and assisted reproductive technology. J Androl. 2004; 25(1):5–18.
Leyens G, Verhaeghe B, Landtmeters M, Marchandise J, Knoops B, Donnay I. Peroxiredoxin 6 is upregulated in bovine oocytes and cumulus cells during in vifro maturation:role of intercellular communication. Biol Reprod. 2004; 71(5):1646–1651.
Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reprod Suppl. 2001; 121(5):647–653.
Lapenna D, Cuccurullo F. Hypochlorous acid and its phaimacolo-gical antagonism:an update picture. Gen Pharmacol. 1996; 27(7):1145–1147.
Vissers MC, Winterboum CC. Oxidation of intracellular glutathione after exposure of human red blood cells to hypochlorous acid. Biochem J. 1995; 307(1):57–62.
Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants:exposure and impact on female fertility. Hum Reprod Update. 2008; 14(4):345–357.
Agarwal A, AUamaneni SSR. Oxidants and antioxidants in human fertility. Middle East Fertil Soe J. 2004; 9(3):187–197.
Diaz FJ, O’Brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary:development of competence to undergo expansion. Dev Biol. 2006; 299(1):91–104.
Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-8 in the mammalian ovary. Mol Endocrinol. 1999; 13(6):1035–1048.
Vanderhyden BC, Macdonald EA, Nagyova E, Dhawan A. Evaluation of members of the TGFbeta superfamily as candidates for the oocyte factors that control mouse cumulus expansion and steroidogenesis. Reproduction. 2003; 61:55–70.
Wang Q, Frolova AI, Purcell S, et al. Mitochondrial dysfunction and apoptosis in cumulus cells of type I diabetic mice. PLoS One. 2010; 5(12):e15901.
Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility - a clinician’s perspective. Reprod Biomed Online. 2005; 11(5):641–650.
Fatehi AN, Roelen BA, Colenbrander B, et al. Presence of cumulus cells during in vitro fertilization protects the bovine oocyte against oxidative stress and improves first cleavage but does not affect further development. Zygote. 2005; 13(2):177–185.
Galeati G, Modina S, Lauria A, Mattioli M. Follicle somatic cells influence pig oocyte penetrability and cortical granule distribution. Mol Reprod Dev. 1991; 29(1):40–46.
Wongsrikeao P, Kaneshige Y, Ooki R, et al. Effect of the removal of cumulus cells on the nuclear maturation, fertilization and development of porcine oocytes. Reprod Domest Anim. 2005; 40(2):166–170.
Wongsrikeao P, Otoi T, Murakami M, et al. Relationship between DNA fragmentation and nuclear status of in vitro-matured porcine oocytes:role of cumulus cells. Reprod Fertil Dev. 2004; 16(8):773–780.
Gutteridge JM, Halliwell B. Comments on review of Free Radicals in Biology and Medicine, second edition. Free Radical Biology and Medicine. 1992; 12(1):93–85.
Sakellariou GK, Vasilaki A, Palomero J, et al. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal. 2013; 18(6):603–621.
Khan SN, Shaeib F, Najafi T, et al. Diffused Intra-oocyte Hydrogen peroxide activates myeloperoxidase and deteriorates oocyte quality. PLoS One. 2015; 10(7):0132388
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaeib, F., Khan, S.N., Ali, I. et al. The Defensive Role of Cumulus Cells Against Reactive Oxygen Species Insult in Metaphase II Mouse Oocytes. Reprod. Sci. 23, 498–507 (2016). https://doi.org/10.1177/1933719115607993
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719115607993