Abstract
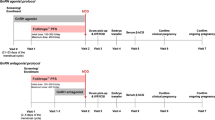
It has been reported that 10% to 15% of young normogonadotrophic women show suboptimal response to standard gonadotropin-releasing hormone—a long protocol. These patients require higher doses of exogenous follicle-stimulating hormone (FSH). This phenomenon could be associated with genetic characteristics. In this study, FSH receptor polymorphism was retrospectively evaluated in 42 normoresponder young women undergoing an in vitro fertilization/intracytoplasmic sperm injection cycle; patients were stratified according to recombinant human FSH (r-hFSH) consumption. We selected 17 normoresponder young patients who required a cumulative dose of recombinant FSH (rFSH) >2500 UI (group A). A control group was randomly selected among patients who required a cumulative dose of rFSH <2500 UI (group B). Follicle-stimulating hormone receptor (FSH-R) 307Ala and 680Ser variants were analyzed in all our patients. Our results show that the mean number of rFSH vials (36.3 ± 7.5 vs 28.6 ± 4.5, P = .0001) and days of stimulation (12.7 ± 2.4 vs 10.8 ± 2.8, P = .03) were significantly lower in group B, whereas the number of oocytes retrieved (7.1 ± 1.5 vs 9.6 ± 2.4; P = .0005) and the average number of embryos transferred (2.1 ± 0.7 vs 2.7 ± 0.4; P = .001) were significantly lower in group A. Estradiol serum levels on the human chorionic gonadotrophin day were significantly lower in group A (997.8 ± 384.9 pg/mL vs 1749.1 ± 644.4; P = .0001). The incidence of the Ser/Ser genotype was higher in patients with higher r-hFSH consumption (group A; P = .02). Based on our results, we hypothesize an association between the FSH-R polymorphisms and a “hyporesponse” to exogenous FSH.
Similar content being viewed by others
References
Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616–1624.
Gizzo S, Andrisani A, Esposito F, et al. Ovarian reserve test: an impartial means to resolve the mismatch between chronological and biological age in the assessment of female reproductive chances. Reprod Sci. 2014;21(5):632–639.
Ferraretti AP, Gianaroli L, Magli MC, D’angelo A, Farfalli V, Montanaro N. Exogenous luteinizing hormone in controlled ovarian hyperstimulation for assisted reproduction techniques. Fertil Steril. 2004;82(6):1521–1526.
De Placido G, Alviggi C, Perino A, et al.; Italian Collaborative Group on Recombinant Human Luteinizing Hormon. Recombinant human LH supplementation versus recombinant human FSH (rFSH) step-up protocol during controlled ovarian stimulation in normogonadotrophic women with initial inadequate ovarian response to rFSH. A multicentre, prospective, randomized controlled trial. Hum. Reprod. 2005;20(2):390–396.
De Placido G, Mollo A, Alviggi C, et al. Rescue of in vitro fertilisation cycles by human menopausal gonadotrophinin pituitary down-regulated normogonadotrophic young women characterised by a poor initial response to recombinant follicle stimulating hormone. Hum Reprod. 2001;16(9):1875–1879.
Devroey P, Fauser BC, Diedrich K; Evian Annual Reproduction (EVAR) Workshop Group 2008. Approaches to improve the diagnosis and management of infertility. Hum Reprod Update. 2009;15(4):391–408.
Alviggi C, Clarizia R, Mollo A, Ranieri A, De Placido G. Who needs LH in ovarian stimulation?. Reprod Biomed Online. 2011;12( Suppl 1):599–607.
Alviggi C, Pettersson K, Longobardi S, et al. A common polymorphic allele of the LH beta-subunit gene is associated with higher exogenous FSH consumption during controlled ovarian stimulation for assisted reproductive technology. Reprod Biol Endocrinol. 2013;11:51.
Huhtaniemi I, Jiang M, Nilsson C, Pettersson K. Mutations and polymorphisms in gonadotropin genes. Mol Cell Endocrinol. 1999;151(1–2):89–94.
Haavisto AM, Pettersson K, Bergendahl M, Virkamäki A, Huhtaniemi I. Occurrence and biological properties of a common genetic variant of luteinizing hormone. J Clin Endocrinol Metab 1995;80(4): 1257–1263.
Alviggi C, Clarizia R, Pettersson K, et al. A common LH polymorphism (LH-beta variant) is associated with suboptimal response to GnRH-a long protocol in normogonadotrophin women undergoing ovarian stimulation with r-hFSH. Reprod Biomed Online. 2009;18(1):9–14.
Alviggi C, Clarizia R, Pettersson K, et al. Suboptimal response to GnRHa long protocol is associated with a common LH polymorphism. Reprod Biomed Online. 2011;22 Suppl 1:S67–S72. doi: 10.1016/S1472-6483(11)60011–4
De Placido G, Alviggi C, Mollo A, et al. Effects of recombinant LH (rLH) supplementation during controlled ovarian hyperstimulation (COH) in normogonadotrophic women with an initial inadequate response to recombinant FSH (rFSH) after pituitary down regulation. Clin Endocrinol. 2004;60(5):637–643.
Alviggi C, Mollo A, Clarizia R, De Placido G. Exploiting LH in ovarian stimulation. Reprod Biomed Online. 2006;12(2):221–233.
Gizzo S, Andrisani A, Noventa M, et al. Recombinant LH supplementation during IVF cycles with a GnRH-antagonist in estimated poor responders: A cross-matched pilot investigation of the optimal daily dose and timing. Mol Med Rep. 2015;12(3):4219–4229.
Perez Mayorga M, Gromoll G, Behre HM, Gassner C, Nieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptore genotype. J Clin Endocrinol Metab. 2000;85(9):3365–3369.
Mohiyiddeen L, Nardo LG. Single-nucleotide polymorphisms in the FSH receptor gene and ovarian performance: future role in IVF. Hum Fertil (Camb). 2010;13(2):72–78.
Yao Y, Ma CH, Tang HL, Hu YF. Influence of folliclestimulating hormone receptor (FSHR) Ser680Asn polymorphism on ovarian function and in-vitro fertilization outcome: a metaanalysis. Mol Genet Metab. 2011;103(4):388–393.
Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18(6):739–773.
Greb R, Grieshaber K, Gromoll J, et al. A common single nucleotide polymorphism in exon 10 of the human follicle stimulating hormone receptor is a major determinant of length and hormonal dynamic of the mestrual cycle. J Clin Endocrinol Metab. 2005;90(8):4866–4872.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821.
Patrelli TS, Berretta R, Gizzo S, et al. CA 125 serum values in surgically treated endometriosis patients and its relationships with anatomic sites of endometriosis and pregnancy rate. Fertil Steril. 2011;95(1):393–396.
De Castro F, Ruiz R, Monitoro R, et al. Role of folliclestimulating hormone receptor Ser680Asn polymorphism in the efficacy of follicle-stimulating hormone. Fertil Steril. 2003;80(3):571–576.
Behre HM, Greb RR, Mempel A, et al. Significance of a common single nucleotide polymorphism in exon 10 of the folliclestimulating hormone (FSH) receptor gene for the ovarian response to FSH: a pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics. 2005;15(7):451–456.
Loutradis D, Patsoula E, Minas V, et al. FSH receptor gene polymorphisms have a role for different ovarian response to stimulation in patients entering IVF/ICSI-ET programs. J Assist Reprod Genet. 2006;23(4):177–184.
Genro VK, Matte U, De Conto E, Cunha-Filho JS, Fanchin R. Frequent polymorphism of FSH receptor do not influence antral follicle responsiveness to follicle-stimulating hormone administration as assessed by the Follicular Output RaTe (FORT). J Assist. Reprod. Genet. 2012;29(7):657–663.
Jun JK, Yoon JS, Ku SY, et al. Follicle stimulating hormone receptor gene polymorphism and ovarian responses to controlled ovarian hyperstimulation for IVF-ET. J Hum Genet. 2006;51(8):665–670.
Gizzo S, Quaranta M, Andrisani A, et al. Serum stem cell factor assay in elderly poor responder patients undergoing IVF: a new biomarker to customize follicle aspiration cycle by cycle. Reprod Sci. 2016;23(1):61–68.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alviggi, C., Conforti, A., Caprio, F. et al. In Estimated Good Prognosis Patients Could Unexpected “Hyporesponse” to Controlled Ovarian Stimulation be Related to Genetic Polymorphisms of FSH Receptor?. Reprod. Sci. 23, 1103–1108 (2016). https://doi.org/10.1177/1933719116630419
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719116630419