Abstract
In the present study, removal of metal ions Pb (II) using nanostructured γ-alumina was investigated in a fixed-bed column. Comparison of Thomas, Yoon-Nelson, and Adams-Bohart models with experimental kinetic results was done, and model parameters were evaluated by linear regression analysis for Pb2+ adsorption in different bed heights, initial concentrations, and flow rates. The obtained experimental data are in good agreement with Thomas and Yoon-Nelson models, but in the case of Adams-Bohart model, low correlation coefficient is observed.
Similar content being viewed by others
Background
Industrial wastewater polluted with heavy metals has endangered the human health and environment. Lead is one of the most extensive toxic metals in the environment [1], so this study has discussed on the continuous flow removal of lead from aqueous solution.
A number of conventional methods for the removal of heavy metals from aqueous solutions have been reported such as precipitation as hydroxides, carbonates, or sulfides; subsequent liquid–solid separation by gravity settling; flotation; ultra-filtration; coagulation; reverse osmosis; ion exchange; electrodialysis; liquid-liquid extraction; membrane processes; evaporation; and biological treatment.
Most of these methods have disadvantages such as high-cost devices, toxic waste production, low efficiency, and require high energy and space. Adsorption process has been shown as an alternative economic method for the removal of heavy metals from aqueous solutions [2–9].
A number of materials have also been used to remove heavy metals from wastewater, such as activated carbon [10], silica [11], titanium dioxide [11], calcium carbonate [11], alumina [11, 12], and recently, various nanomaterials such as nanometal oxides [13], carbon nanotubes [14], and nanozeolite composites [15].
Nanoalumina has several crystalline form and mainly used as γ-alumina form.
By considering the γ-alumina, surface area is in the range of 150 to 500 m2/g and pore radius is in the range of 1.5 to 6 nm, so γ -alumina is anticipated to be more adsorptive active than α-alumina. γ-Al2O3 nanoparticles is a promising material as an adsorbent because of its large specific surface area, high adsorption capacity, mechanical strength, and low-temperature modification. Nanoalumina is used widely as electrical insulator, catalyst, and base catalyst in many chemical reactions, membrane processes, microelectronics, and water and wastewater purification [16–21].
Unuabonah et al. studied in polymer-clay-based composite adsorbent to remove lead (II) ions from aqueous solution in a fixed-bed mode. The increase in bed height and initial metal ion concentration increased the adsorption capacity of lead (II). The Yoon-Nelson, Thomas, and Clark models were found to give good fit to adsorption data. On the other hand, Adams-Bohart was found to be a poor predictor for the column operation [22].
Kundu and Gupta studied in continuous fixed bed to evaluate the efficiency of iron oxide-coated cement (IOCC) as an adsorbent for the removal of As(III) from aqueous solution under the effect of various process parameters like bed depth, flow rate, and initial As(III) concentrations. The dynamics of the adsorption process was modeled by bed depth service time (BDST), mass transfer, Thomas, and Yoon-Nelson models. The Thomas and Yoon-Nelson model predictions were in very good agreement with the experimental results at all the process parameters studied, indicating that they were very suitable for IOCC column design [23].
Rao et al. studied in Syzygium cumini L leaf powder as a biosorbent for generating adsorption data in a fixed-bed minicolumn. Effect of flow rate, initial Cd (II) concentration, and bed height was the experimental parameters chosen to obtain breakthrough curves. Adams-Bohart, BDST, Thomas, and Yoon-Nelson models were applied to the data for predicting breakthrough curves and to determine the characteristic parameters. Prominent and unique characteristic features of the respective models like service time (Hutchins BDST model), adsorption capacity (Thomas model), and time required for 50% breakthrough (Yoon-Nelson model) were determined [24].
Han et al. studied in continuous adsorption in a fixed-bed column using phoenix tree leaf powder as an adsorbent for the removal of methylene blue (MB) from aqueous solution. The effect of flow rate, influent MB concentration, and bed depth on the adsorption characteristics of adsorbent was investigated at pH 7.4. Four kinetic models, Thomas, Adams-Bohart, Yoon-Nelson, and Clark, were applied to experimental data to predict the breakthrough curves by nonlinear regression and to determine the characteristic parameters of the column that are useful for process design [25].
Purpose of this study is removal of Pb2+ by nanostructured γ-alumina using fixed bed with upward flow. Effects of parameters such as flow rate, initial concentration of lead, and fixed bed height on lead adsorption using Thomas, Yoon-Nelson, and Adams-Bohart kinetic models were considered.
Materials
Nanostructured γ-alumina prepared from nano Pars Lima Co. (Tehran, Iran) as adsorbent and lead nitrate as adsorbate were obtained from Merck C. (Germany). All materials were prepared with high purity. Double distilled water was used. Dilute HNO3 and NaOH were used to adjust pH.
Atomic absorption spectrophotometer with acetylene flame (PG 990) was used for the measurement of lead ions; digital pH meter (Sartorius (PB-11)) was used to measure pH. Different flow rates were performed using a peristaltic pump ETATRON DS (Rome, Italy) that is capable to adjust flow rates in milliliter-per-minute range.
Continuous flow studies
Thomas model
The Thomas model is widely used in column performance modeling. Its derivation assumes Langmuir kinetics of adsorption-desorption and no axial dispersion. The expression for the Thomas model for adsorption column is given as Equation 1 [26]:
where kTh (mL/min.mg) is the Thomas model constant, qe (mg/g) is the predicted adsorption capacity, x is mass of adsorbent (g), Q is influent flow rate (mL/min), C0 is initial solution concentration (mg/L), and C t is effluent solution concentration (mg/L). The linear form of Thomas model is expressed as Equation 2:
Yoon-Nelson model
This model is simpler than other models and also requires no data about the characteristics of the system such as the type of adsorbent and the physical properties of the adsorption bed.
The Yoon-Nelson equation is expressed as Equation 3 [27]:
where kYN (1/min) is the rate constant and τ (min) is the time required for 50% adsorbate breakthrough. The linear form of Yoon-Nelson model is expressed as Equation 4:
Adams-Bohart model
The Adams-Bohart model is based on the assumption that the rate of adsorption is proportional to both the concentration of the adsorbing species and the residual capacity of the adsorbent. The Adams-Bohart model is only used for the description of the initial part of the breakthrough curve and is expressed as Equation 5 [28]:
where kAB (l/min.mg) is rate constant of Adams-Bohart model, z (cm) is the bed depth, N0 (mg/L) is maximum ion adsorption capacity per unit volume of adsorbent column, and U0 (cm/min) is the linear velocity of influent solution. The linear form of Adams-Bohart model is expressed as Equation 6:
Methods
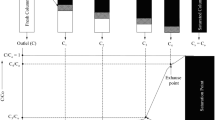
A Pyrex column (Sigma-Aldrich, St. Louis, MO, USA) 30 cm in height and 1 cm in diameter was used with upward flow. In order to avoid the exiting adsorbent from column, glass wool at the top and bottom of the column was applied. Schematic of experimental set-up is shown in Figure 1.
In order to investigate the effect of flow rate on the behavior of the adsorbent column, flow rates 3, 6, and 9 mL/min with constant concentrations 100 ppm, constant bed height 10 cm were used. In order to investigate the effect of height on the behavior of the adsorbent column, heights 5, 10, and 15 cm (equivalent with 3.5342, 7.0685, and 10.6028 gr) with constant concentrations 100 ppm and constant flow rate 6 mL/min were used, and in order to investigate the effect of initial concentration of lead ion on behavior of the adsorbent column, concentrations 65, 100, and 150 ppm with constant bed height 10 cm and constant flow rate 6 mL/min were used. Effluent samples from the column were collected at specified time intervals, and remaining lead concentrations in the solution were measured by atomic absorption technique.
In all experiments, the temperature and pH values were adjusted to 25°C and 4.5, respectively.
Maximum adsorption capacity of the adsorbent in a fixed-bed column is calculated by Equations 7 and 8 [22]:
where qe,max (mg/g) is maximum adsorption capacity of the adsorbent, Q (mL/min) is flow rate, C0 (mg/L) is initial concentration, C t (mg/L) is concentration in specified time, and w (g) is mass of the adsorbent.
Results and discussions
Characterization of adsorbent
Characterization of nanostructured γ-alumina is presented in Table 1.
Evaluation of Thomas model parameters
To determine maximum adsorption capacity of the adsorbent (qe,max) and kinetic coefficient (kTh) in Thomas model, experimental data were fit into the Equation 2. Linear regression results and values of R2 are listed in Table 2, where values of R2 ranged from 0.9109 to 0.9401. According to Table 2, the calculated qe,max from Thomas model is greater than experimental qe,max. It is also seen in Table 2 that as C0 and adsorbent weight increased, the value of qe,max and kTh increased and decreased, respectively. This is because the driving force for adsorption is the difference in concentration between the lead on the adsorbent and in the solution [29]. As flow rate increased, the value of qe,max decreased. This is because of unavailability of reaction sites, but the value of kTh increased. Linear form of Thomas model in different heights, flow rates, and concentrations is shown in Figures 2, 3, and 4, respectively. As can be observed, the experimental data are in good agreement with the theoretical results.
Evaluation of Yoon-Nelson model parameters
Linear regression results and values of R2 are presented in Table 3. It was observed that rate constant (kYN) and time required for 50% adsorbate breakthrough (τ) decreased and increased, respectively, with increasing adsorbent dose. Also, it was found that the rate constant (kYN) increases as much as flow rate increases. Because of less residence time of metal ion in adsorbent bed, time required for 50% adsorbate breakthrough (τ) decreased with increasing flow rate (Table 3). Linear plot of Yoon-Nelson model in different heights, flow rates, and concentrations is shown in Figures 5, 6, and 7, respectively. As can be observed, the experimental data are fit well with the model. It can be concluded that both Thomas and Yoon-Nelson models are appropriate models to describe fixed-bed operations.
Evaluation of Adams-Bohart model parameters
Linear regression results (Table 4) are shown that adsorption capacity of the adsorbent (N0) and kinetic constant of the model (kAB) increased and decreased, respectively, with increasing adsorbent dose and initial lead concentration. Because of more saturation of adsorbent sites, adsorption capacity of the adsorbent (N0) decreased with increasing flow rate. Also, kinetic constant of the model (kAB) increased with increasing flow rate. Linear plot of Adams-Bohart model in different heights, flow rates, and concentrations is shown in Figures 8, 9, and 10, respectively.
The lower R2 values relative to the other models can be interpreted that the Adams-Bohart model is not as appropriate a predictor for the breakthrough curve.
Conclusions
Studies on continuous adsorption using nanostructured γ-alumina to remove Pb (II) indicate that this adsorbent has high ability to remove heavy metal ions from aqueous solutions.
Adsorption capacity strongly depends on flow rate, influent concentration, and bed height.
Comparison of Thomas, Yoon-Nelson, and Adams-Bohart kinetic models with experimental data was performed, and model parameters were determined by linear regression analysis for Pb2+ adsorption under various operating conditions. The experimental data fit well with Thomas and Yoon-Nelson models, but the Adams-Bohart model predicted poor performance of fixed-bed column.
Results showed that with increasing initial concentration of lead, adsorption capacity of the adsorbent and kinetic constant of model increased and decreased, respectively, in the Thomas model. Fifty percent adsorbate breakthrough and rate constant decreased and increased, respectively, in the Yoon-Nelson model. Capacity of the adsorbent and kinetic constant increased and decreased, respectively, in the Adams-Bohart model.
References
Paulino AT, Santos LB, Nozaki J: Removal of Pb2+, Cu2+, and Fe3+ from battery manufacture wastewater by chitosan produced from silkworm chrysalides as a low-cost adsorbent. React. Funct. Polym 2008, 68: 634–642. 10.1016/j.reactfunctpolym.2007.10.028
Marder L, Sulzbach GO, Bernardes AM, Ferreira JZ: Removal of cadmium and cyanide from aqueous solutions through electrodialysis. J. Electrochem. Soc 2003, 14: 610–615.
Bradl HB: Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interf Sci 2004, 277: 1–18. 10.1016/j.jcis.2004.04.005
Zouboulis AI, Matis KA: Removal of metal ions from dilute solutions by sorptive flotation. Crit. Rev. Sci. Tech 1997, 27: 195–235. 10.1080/10643389709388499
Srivastava SK, Gupta VK, Mohan D: Removal of lead and chromium by activated slag-A blast-Furnace waste. J. Envir. Eng 1997, 123: 461–468. 10.1061/(ASCE)0733-9372(1997)123:5(461)
Wang J, Chen C: Biosorbents for heavy metals removal and their future. Biotech. Adv 2009, 27: 195–226. 10.1016/j.biotechadv.2008.11.002
Hota G, Kumar BR, Ng WJ, Ramakrishna S: Fabrication and characterization of a boehmite nanoparticles impregnated electrospun fiber membrane for removal of metal ions. J. Mater. Sci 2008, 43: 212–217. 10.1007/s10853-007-2142-4
Afkhami A, Conway BE: Investigation of removal of Cr(VI), Mo(VI), W(VI), V(IV), and V(V) oxy-ions from industrial waste-waters by adsorption and electrosorption at high-area carbon cloth. J. Colloid Interface Sci 2002, 251: 248–255. 10.1006/jcis.2001.8157
Afkhami A, Madrakian T, Amini A, Karimi Z: Effect of the impregnation of carbon cloth with ethylenediaminetetraacetic acid on its adsorption capacity for the adsorption of several metal ions. J. Hazard. Mater 2008, 150: 408–412. 10.1016/j.jhazmat.2007.04.123
Mohan D, Singh KP: Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse–an agricultural waste. J. Water Res 2002, 36: 2304–2318. 10.1016/S0043-1354(01)00447-X
Kim MS, Chung JG: Removal of copper(II) ion by kaolin in aqueous solutions. Environ. Eng. Res 2002, 7: 49–57.
Genc-Fuhrman H, Mikkelsen PS, Ledin A: Simultaneous removal of As, Cd, Cr, Cu, Ni and Zn from stormwater: experimental comparison of 11 different sorbents. Water Res 2007, 41: 591–602. 10.1016/j.watres.2006.10.024
Zhang L, Huang T, Zhang M, Guo X, Yuan Z: Studies on the capability and behavior of adsorption of thallium on nano-Al 2 O 3 . J. Hazard. Mater 2008, 157: 352–357. 10.1016/j.jhazmat.2008.01.005
Ahmedna M, Marshall WE, Husseiny AA, Rao RM, Goktepe I: The use of nutshell carbons in drinking water filters for removal of trace metals. Water Res 2004, 38: 1062–1068. 10.1016/j.watres.2003.10.047
Ngomsik AF, Bee A, Draye M, Cote G, Cabuil V: Magnetic nano- and microparticles for metal removal and environmental applications: a review. CR Chimie 2005, 8: 963–970. 10.1016/j.crci.2005.01.001
Turker AR: New sorbents for solid-phase extraction for metal enrichment. Clean 2007, 35: 548–557.
Li J, Shi Y, Cai Y, Moua S, Jiang G: Adsorption of di-ethyl-phthalate from aqueous solutions with surfactant-coated nano/microsized alumina. Chem. Eng. J 2008, 140: 214–220. 10.1016/j.cej.2007.09.037
Hiraide M, Iwasawa J, Hiramatsu S, Kawaguchi H: Use of surfactant aggregates form on alumina for the preparation of chelating sorbents. Anal. Sci 1995, 11: 611–615. 10.2116/analsci.11.611
Srivastava V, Weng CH, Singh VK, Sharma YC: Adsorption of Nickel Ions from Aqueous Solutions by Nano Alumina: Kinetic, Mass Transfer, and Equilibrium Studies. J. Chem. Eng. Data 2011, 56: 1414–1422. 10.1021/je101152b
Sharma YC, Srivastava V, Upadhyay SN, Weng CH: Alumina nanoparticles for the removal of Ni(II) from aqueous solutions. Ind. Eng. Chem. Res 2008, 47: 8095–8100. 10.1021/ie800831v
Treybal RE: Mass Transfer Operations. 3rd edition. Mc Graw Hill Book Company, Japan; 1990.
Unuabonah EI, El-Khaiary MI, Olu-Owolabi BI: Predicting the dynamics and performance of a polymer-clay based composite in a fixed bed system for the removal of lead(II) ion. Chem. Eng. Res. Design 2012, 90: 1105–1115. 10.1016/j.cherd.2011.11.009
Kundu S, Gupta AK: As(III) removal from aqueous medium in fixed bed using iron oxide-coated cement (IOCC): experimental and modeling studies. Chem. Eng. J 2007, 129: 123–131. 10.1016/j.cej.2006.10.014
Rao KS, Anand S, Venkateswarlu P: Modeling the kinetics of Cd(II) adsorption on Syzygium cumini L leaf powder in a fixed bed mini column. J. Ind. Eng. Chem 2011, 17: 174–181. 10.1016/j.jiec.2011.02.003
Han R, Wang Y, Zhao X, Wang Y, Xie F, Cheng J, Tang M: Adsorption of methylene blue by phoenix tree leaf powder in a fixed-bed column: experiments and prediction of breakthrough curves. Desalination 2009, 245: 284–297. 10.1016/j.desal.2008.07.013
Thomas HC: Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc 1944, 66: 1466–1664.
Yoon YH, Nelson JH: Application of gas adsorption kinetics. I. A theoretical model for respirator cartridge service time. Am. Ind. Hyg. Assoc. J 1984, 45: 509–516. 10.1080/15298668491400197
Loderio P, Herreo R, Sastre de Vicentes ME: The use of protonated Sargassum muticum as biosorbent for cadmium removal in a fixed-bed column. J. Hazard. Mater 2006, 137: 244–253. 10.1016/j.jhazmat.2006.01.061
Aksu Z, Gonen F: Biosorption of phenol by immobilized activated sludge in a continuous packed bed: prediction of breakthrough curves. Process. Biochem 2004, 39: 599–613. 10.1016/S0032-9592(03)00132-8
Acknowledgments
The authors wish to thank Mr. Bagher Biralvand for his great help in the atomic absorption analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interest.
Authors’ contributions
ZS carried out the preparation of experimental elements. ZS and RS participated in doing the experiments and analyzed, interpreted, and organized the data. RF designed and coordinated the overall project. ZS, RS and RF carried out the manuscript preparation. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Saadi, Z., Saadi, R. & Fazaeli, R. Fixed-bed adsorption dynamics of Pb (II) adsorption from aqueous solution using nanostructured γ-alumina. J Nanostruct Chem 3, 48 (2013). https://doi.org/10.1186/2193-8865-3-48
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-48