Abstract
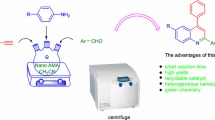
Nano SnO2 catalyzes the two-component reaction of 2-aminobenzophenones with acetylenic mono or diesters under mild conditions to afford quinoline derivatives in high yields. Nano SnO2 shows high activity when used as surface catalyst for the synthesis of quinoline derivatives.
Similar content being viewed by others
Background
Nanocatalysis has an important role in the chemistry of nanoscience [1–4]. Many nanoparticles are used as the materials that can catalyze various chemical reactions. In some cases, nanoparticles are put on the surface since this facilitates recovery of the catalyst [5]. In addition, in several cases, users can increase the selectivity because of the high synergistic interaction between nanoparticle and solid supports [6, 7]. The applications of SnO2 are in the semiconductors, and it has been widely used as gas sensor [8], solar cells [9], lithium battery and use in the materials [10], and catalysts [11]. In the area of nanocatalysis, tin oxide (SnO2) is one of the most extensively studied materials [12]. Quinoline derivatives are one of the most applicable products in the chemistry of anti-hypertensive and anti-inflammatory, with tyrosine-kinase PDGf-RTK as an inhibitor agent [13–21]. Due to these uses, there has been an increasing interest in the development of quinoline synthesis. There are various general methods for the synthesis of this heterocycle such as Skraup, Friedlandear, Dobner-von Miller, Combes, Pfitzinger, and Conrad-Limpach [22–26]. These classical syntheses are frequently used for the preparation of pharmaceutical agents, ligands, and functional materials containing a quinoline skeleton [27]. Previously, we have reported the synthesis of 4-arylquinolines via a three-component reaction of acetylenic esters and 2-aminobenzophenone derivatives in the presence of triphenylphosphine (Scheme 1) [28].
Methods
1H NMR spectra were recorded on DRX-400AVANCE spectrometer, Bruker AXS, Inc., Karlsruhe, Germany, at 400 MHz; infrared (IR) spectra were recorded on a Shimadzu IR-470 spectrometer, Kyoto, Japan. Nano SnO2 powder was achieved from NanoPac Persia Co., Isfahan, Iran. Thin layer chromatography (TLC) was carried out on Fluka Silica gel TLC- cards, St. Louis, MO, USA. All of the other reagents and solvents were used as received from commercial suppliers. A transmission electron microscope (TEM) (ZEISS EM900 operating at 50 kV, Carl Zeiss AG, Germany) was used to observe the morphology and size of the nanoparticles. The XRD pattern was acquired using a D8 ADVANCE, Bruker. The diffractograms were measured for 2θ, in the range of 10 to 80°, using Cu Kα incident beam (λ = 1.51418 Å).
General procedure for the preparation of 4-arylquinolines (exemplified by 3a)
To a stirred mixture of 2-aminobenzophenone (2 mmol) and acetylenic ester (2 mmol) in EtOAc, 5 ml SnO2 nanoparticles (5 mol%) was added. The reaction mixture was refluxed for 1 h. The progress of the reaction was monitored by TLC. After completion of the reaction, the solid product was filtered and purified by dissolving in chloroform, and the nanocatalyst was removed by a centrifuge and pure product 3a was obtained by removing the solvent. The nanocatalyst was recovered to be reused in subsequent reactions without losing any activity. The structure of the product was confirmed by melting point, IR, and 1H NMR spectra. The results were compared with authentic samples.
-
Dimethyl 4-phenyl-2,3-quinolinedicarboxylate (3a): White powder; yield: 90%. mp 122°C to 124°C. IR (KBr) (υmax/cm−1): 3,055 (C sp2 -H), 2,994 (C sp3 -H), 1,725 (CO); 1H NMR (400 MHz, CDCl3): δH 3.61 (s, 3H, OCH), 4.04 (s, 3H, OCH), 7.34 (dd, 3JHH = 6.4 Hz, 3JHH = 2.0 Hz, 2H, 2CH), 7.47 (d, 3JHH = 2.0 Hz, 2H, 2CH), 7.48 (t, 3JHH = 6.4 Hz, 1H, CH), 7.55 (t, 3JHH = 7.7 Hz, 1H, CH), 7.62 (d, 3JHH = 7.7 Hz, 1H, CH), 7.79 (ddd, 3JHH = 8.5 Hz, 3JHH = 7.7 Hz, 4JHH = 1.4 Hz, 1H, CH), 8.3 (d, 3JHH = 8.5 Hz,1 H, CH).
-
Diethyl 4-phenyl-2,3-quinolinedicarboxylate (3b): White powder; yield: 87%. mp 96°C to 98°C. IR (KBr) (υmax/cm−1): 3,032 (C sp2 -H), 2,985 (C sp3 -H), 1,728(CO); 1H NMR (400 MHz, CDCl3): δH 0.96 (t, 3JHH = 7.1 Hz, H, CH3 ), 1.43 (t, 3JHH = 7.1 Hz, 3H, CH3), 4.06 (q, 3JHH = 7.1 Hz, 2H, OCH2), 4.50 (q, 3JHH = 7.1 Hz, 2H), 7.33 (dd, 3JHH = 6.5 Hz, 3JHH = 2.2 Hz, 2H, 2CH), 7.45 (d, 3JHH = 2.2 Hz, 2H, 2CH), 7.46 (t, 3JHH = 6.5 Hz, 1H, CH), 7.53 (t, 3JHH = 7.8 Hz, 1H, CH), 7.59 (d, 3JHH = 7.8 Hz, 1H, CH), 7.79 (ddd, 3JHH = 8.5 Hz, 3JHH = 7.8 Hz, 4JHH = 1.4 Hz, 1H, CH), 8.29 (d, 3JHH = 8.5 Hz, 1 H, CH).
-
Methyl 4-phenyl-2-quinolinecarboxylate (3c): White powder; yield: 60%. mp 101°C to 103°C. IR (KBr) (υmax/cm−1): 3,010 (C sp2 -H), 2,995 (C sp3 -H), 1,725 (CO); 1H NMR (400 MHz, CDCl3): δH 4.07 (s, 3H, OCH3), 7.49 to 7.54 (m, 5H, -Ph), 7.58 (dd, 3JHH = 8.3, 3JHH = 7.7 Hz, JHH = 1.1 Hz, 1H, CH), 7.7 (ddd, 3JHH = 8.4 Hz, 3JHH = 7.7 Hz, 4JHH = 1.4 Hz, 1H, CH), 7.96 (d, 3JHH = 8.3 Hz, 1H, CH), 8.14 (s, 1H, CH3), 8.36 (d, 3JHH = 8.4 Hz, 1H, CH).
-
Ethyl 4-phenyl-2-quinolinecarboxylate (3d): White powder; yield: 65%. mp 125°C to 127°C. IR (KBr) (υmax/cm−1): 3,045 (C sp2 -H), 2,985 (C sp3 -H), 1,724 (CO).
-
Dimethyl 6-chloro-4-phenyl-2,3-quinolinedicarboxylate (3e): White powder; yield: 80%. mp 158°C to 160°C. IR (KBr) (υmax/cm−1): 3,074 (Csp2-H), 2,958 (Csp3-H), 1,732 and 1,729 (CO).
-
Diethyl 6-chloro-4-phenyl-2,3-quinolinedicarboxylate (3f): White powder; yield: 90%. mp 163°C to 165°C. IR (KBr) (υmax/cm−1): 3,075 (C sp2 -H), 2,988 (C sp3 -H), 1,739 and 1,724 (CO).
-
Methyl 6-chloro-4-phenyl-2-quinolinecarboxylate (3g): White powder; yield: 70%. mp 177°C to 179°C. IR (KBr) (υmax/cm−1): 3,054 (C sp2 -H), 2,998 (C sp3 -H), 1,719 (CO).
-
Ethyl 6-chloro-4-phenyl-2-quinolinecarboxylate (3h): Yellow powder; yield: 75%. mp 171°C to 173°C. IR (KBr) (υmax/cm−1): 3,064 (C sp2 -H), 2,983 (C sp3 -H), 1,729 (CO).
-
Dimethyl 6-chloro-4-(2-chlorophenyl)-2,3-quinolinedicarboxylate (3i): Yellow powder; yield: 74%. mp 190°C to 192°C. IR (KBr) (υmax/cm−1): 3,070 (C sp2 -H), 2,985 (C sp3 -H), 1,729 and 1,720 (CO).
-
Diethyl 6-chloro-4-(2-chlorophenyl)-2,3-quinolinedicarboxtlate (3j): White powder; yield: 87%. mp 127°C to 129°C. IR (KBr) (υmax/cm−1): 3,074 (C sp2 -H), 2,983 (C sp3 -H), 1,729 and 1,724 (CO).
-
Dimethyl 6-chloro-4-(2-fluorophenyl)-2,3-quinolinedicarboxylate (3k): White powder; yield: 92%. mp 210°C to 212°C. IR (KBr) (υmax/cm−1): 3,079 (C sp2 -H), 2,953 (C sp3 -H), 1,729 and 1,718 (CO).
-
Diethyl 6-chloro-4-(2-fluorophenyl)-2,3-quinolinedicarboxylate (3l): White powder; yield: 95%. mp 135°C to 137°C. IR (KBr) (υmax/cm−1): 3,084 (Csp2-H), 2,983 (Csp3-H), 1,729 and 1,719 (CO).
Results and discussion
In this work, we report a methodology reaction in which 2-aminobenzophenones 2 react with acetylenic mono or diesters 1 in the presence of nano SnO2 (Scheme 2).
The yields of the two component reactions show that nano SnO2 catalyzes these reactions efficiently (Table 1). It was found that nano SnO2 acts as both site of catalysis and a support for this system due to their high surface area. A possible mechanism of the reaction is shown in Scheme 3. It is reasonable to assume that the initial attack of nucleophilic amine to the acetylenic ester leads to the formation of intermediate 4 on the surface of nano SnO2 which can act as Lewis acid to increase the electrophilicity of the carbonyl group of 2-aminobenzophenone. Then, the product 3 is formed by loss of one molecule of water under reflux condition (Scheme 3).
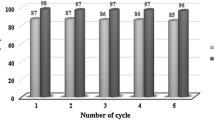
In the procedure of optimizing the reaction conditions, we monitored the solvent effect, temperature dependency, and the amount of nanocatalyst. To study the optimized condition of the reaction parameters, the reaction between 2-aminobenzophenone and DMAD was considered as the model reaction. The effect of different solvents was monitored, and the excellent results were obtained when the reaction has been carried out in EtOAc. The temperature monitoring showed that the best temperature for this reaction is the reflux condition for 1 h. Also, when the reaction was scaled up to 5 mol% of nano SnO2, the highest yield was achieved (Table 2), and in the absence of SnO2 catalyst no formation of the expected product was detected even after 3 h.
The morphology, crystal structure, and sizes of the SnO2 nanocatalyst were characterized by X-ray diffraction and TEM analyses. The powder X-ray diffraction pattern of the reaction mixture was recorded on a Rigaku D/Max-2200 diffractometer, Shibuya-ku, Japan, equipped with horizontal goniometer in θ/2θ geometry. The Cu Kα (l = 1.5418 Å) radiation was used, and the sample was scanned between 3° to 80° 2θ. The sharp peaks in the diffractogram indicate that the reaction media (nano SnO2 in the dimethyl 4-phenyl-2,3-quinolinedicarboxylate) contain crystalline species. There are several major peaks with 2θ values of 26.63, 33.91, 37.94, 42.61, 51.80, 54.77, and 57.83, corresponding to SnO2 crystal planes of (1 0 1), (1 1 0), (1 1 1), (2 1 0), (2 1 1), (2 2 0), and (0 0 2), respectively. All reflections of SnO2 nanoparticles show tetragonal rutile structure (JCPDS 41–1445).
TEM images of the SnO2 nanocatalyst in the reaction mixture (nano SnO2 in the dimethyl 4-phenyl-2,3-quinolinedicarboxylate) in different scales (50, 100, 150, 200, and 300 nm) were obtained. Also, using the bright field technique, the nanoparticles were observed; the nanoparticles look distinctly darker than the organic phase due to the much higher electron density, the nanoparticles clearly distinguishable from organic media. The obtained pictures showed that the nanoparticles are in good dispersion state, and the nanoparticles are in the range of 70 to 95 nm.
Conclusions
In conclusion, we have reported an efficient procedure for the synthesis of quinoline derivatives using SnO2 nanoparticles as a non-toxic and inexpensive heterogeneous nanocatalyst. The method offers advantages such as ease of the work-up, low loading of catalyst, high yields of products and short reaction times.
References
Corma A, Serna P: Chemoselective Hydrogenation of Nitro Compounds with Supported Gold Catalysts. Science 2006, 313: 332.
Enache DI, Edwards JK, Landon P, Solsona-Espriu B, Carley AF, Herzing AA, Watanabe M, Kiely CJ, Knigh DW, Hutchings GJ: Science. 2006, 311: 362.
Hughes MD, Xu J, Jenkins P, McMorn P, Landon P, Enache DI, Carley AF, Attard GA, Hutchings GJ, King F, Stitt EH, Johnston P, Griffin K, Kiely CJ: Nature. 2005, 437: 1132.
Liang HP, Zhang HM, Hu JS, Guo YG, Wan YLJ, Bai CL: Angew. Chem. Int. 2004, 43: 1540.
Astruc D, Lu F, Aranzaes JR: Angew. Chem. Int. Ed.. 2005, 44: 7852.
Valden M, Lai X, Goodman DW: Science. 1998, 281: 1647.
Huang J, Jiang T, Gao HX, Han BX, Liu ZM, Wu WZ, Chang YH, Zhao GY: Angew. Chem. Int. Ed.. 2004, 43: 1397.
Li C, Bi L: F. Shaming. J. Rare Earths.. 2007, 25: 505.
El-Etre AY, Reda SM: Appl. Surf. Sci.. 2010, 256: 6601.
Li C, Wei W, Fang S, Wang H, Zhang Y, Gui Y: R. Chen. J. Power Sources.. 2010, 195: 2939.
Arpita S, Sudip KG, Panchanan P: J. Mol. Catalysis A: Chemical.. 2010, 327: 73.
Modou MJ, Roy S: Morrison Chemical Sensing with Solid State Devices. Academic Press, New York; 1989.
Barton D, Ollis WD: Comprehensive organic chemistry: the synthesis and reaction of organic compounds, vol 4. Pergamon, Oxford; 1979:166–203.
Desai PK, Desai P, Machhi D, Desai CM, Patel D: Indian J. Chem.. 1996, 35: 871.
Sonza MV, Vasconcelos TPA: Quim. Nova.. 2005, 28: 678.
Hirano Y, Uehara M, Saeki K, Kato T, Takahashi K, Mizutani T: J. Health. Sci.. 2002, 48: 118.
Larsen RD, Corley EG, King AO, Carrol JD, Davis P, Verhoevea TR, Reider PJ, Lablle M, Ganthier JY, Xiang YB, Zamboni RJ: J. Org. Chem.. 1996, 61: 3398.
Chen YL, Fang KC, Sheu JY, Hsu SL, Tzeng CC: J. Med. Chem.. 2001,44(K.C Fang):2374.
Kallmaya B, Serrnivasa S: Farmaco. 1998, 53: 399.
Ko TC, Hour MJ, Liem JC, Teng CM, Lee KH, Kno SC, Huang L: J. Bio. Org. Med. Chem. Lett. 2001, 11: 279.
Ferrarini PL, Mori C, Badwneh M, Menera C, Martinelli A, Miceli M, Ramagnoli F, Saccomanui G: J. Heterocycl. Chem. 1997, 34: 1501.
Maguire MP, Sheets KR, Mcverty K, Spada AP, Zilberstain A: J. Med. Chem.. 1994, 37: 2129.
Bergstrom FW: Chem. Rev.. 1944, 35: 77.
Edward A: J. Org. Chem.. 1966, 31: 2899.
Jones G: The Chemistry of Heterocyclic Compounds, vol. 32, part I. Edited by: Weissberger A, Taylor EC. Wiley, Chichester; 1997:93–318.
Reitsema RH: Chem. Rev.. 1948, 43: 43.
Jones G: Comprehensive Heterocyclic Chemistry, vol. 5, chap. 5. Edited by: Kat-ritzky AP, Rees CW, Scriven EE. Pergamon, Oxford; 1996:167.
Asghari S, Qandalee M, Naderi Z: Z. Sobhaninia. Mol. Divers.. 2010, 14: 569.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Qandalee, M., Alikarami, M., Mighani, H. et al. Synthesis of quinoline derivatives from the reaction of aminobenzophenones and acetylenic esters in the presence of SnO2 nanoparticles. Int Nano Lett 3, 49 (2013). https://doi.org/10.1186/2228-5326-3-49
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-3-49