Abstract
Background
Ficus carcia leaves (fig leaves) for the removal of cadmium(II) and lead(II) ions from aqueous solutions have been investigated.
Results
The biosorption of cadmium(II) and lead(II) ions was found to be dependent on the solution pH, initial metal ion concentrations, biosorbent dose, contact time, and temperature. The experimental equilibrium biosorption data were analyzed by two widely used two parameters, Langmuir and Freundlich isotherm models.
Conclusions
Langmuir isotherm model provided a better fit with the experimental data than Freundlich model by high correlation coefficients R2. Kinetic studies showed that pseudo-second-order described the biosorption experimental data better than the pseudo-first-order kinetic model.
Similar content being viewed by others
Background
Cadmium(II) and lead(II) ions have been released to the environment through phosphate fertilizers, electrical wiring, manufacturing batteries, air conditioning tubing, plumbing, electroplating, and pigments. There is a growing demand to find relatively efficient, low-cost, and easily available adsorbents for the adsorption of toxic heavy metals. Different agrowastes have been investigated for the biosorption of Cd(II) and Pb(II) ions such as Caladium bicolor[1], neem leaf [2]; heartwood powder of Areca catechu[3]; Ulmus carpinifolia and Fraxinus excelsior tree leaves [4]; green coconut shell powder [5]; mango peel waste [6]; banana, lemon, and orange peel [7]; chemically modified Moringa oleifera tree leaves [8]; maize leaf [9]; Scolymus hispanicus[10]; processed walnut shell [11]; sugar cane residue or bagasse [12]; pomegranate peel [13], walnut, hazelnut, almond, pistachio, and apricot stone [14]; Iagenaria vulgaris shell [15]; grape stalk waste [16]; rice husk [17]; paper mulberry [18]; Eucalyptus camaldulenis tree leaves [19]; Saraca indica leaf powder [20]; and olive tree pruning waste [21].
In the present work, we have studied the potential of cadmium(II) and lead(II) biosorption on an agromaterial which Ficus carcia leaves (FL) coming from fig fruit tree waste. Results from this study can be used to assess the utility of FL for cadmium(II) and lead(II) removal from aqueous solution.
Methods
Preparation of biosorbent
The raw FL were collected from a local plantation in Jordan. This agricultural waste was thoroughly rinsed with water to remove dust and soluble material. Then it was allowed to dry at room temperature. The dried leaves were grounded to a fine powder in a grinding mill (Retsch RM 100, Thermo Fisher Scientific, NH, USA) and sieved to get size fraction <44 μm and then dried in an oven at 60°C for 4 h.
Preparation of Cd(II) and Pb(II) stock solutions
All the chemicals used were of analytical reagent (AR) grade purchased from Merck, Germany. Stock solutions of 1,000 mg/L of cadmium(II) and lead(II) were prepared from cadmium nitrate [Cd (NO3)2 · 4H2O] and lead nitrate [Pb (NO3)2], respectively, using double distilled water. Desired test solutions of Cd(II) and Pb(II) ions were prepared using appropriate subsequent dilutions of the stock solution. The range of concentrations of Cd(II) and Pb(II) ions prepared from standard solution varies between 10 and 100 mg/L. Before mixing the adsorbent, the pH of each test solution was adjusted to the required value with 0.1 M NaOH or 0.1 M HCl.
Analysis
The concentrations of Cd(II) and Pb(II) ions in the solutions before and after equilibrium were determined by AAS6300 atomic absorption spectrometer (Shimadzu, Japan). The pH of the solution was measured with a WTW pH meter using a combined glass electrode. Fourier transform infrared spectroscopy (FTIR) IR Prestige-21 (Shimadzu, Tokyo, Japan) was used to identify the different chemical functional groups present in the FL. FTIR analyses were also used to determine the functional groups which are responsible for the metal binding with FL. The analysis was carried out using KBr, and the spectral range varies from 4,000 to 400 cm−1.
Biosorption experiments
Batch biosorption experiments were conducted by mixing biosorbent with Cd(II) or Pb(II) ion solutions with desired concentration in 250 mL glass flask. The glass flasks were stoppered during the equilibration period and placed on a temperature-controlled shaker at a speed 120 rpm. The amount of biosorption was calculated based on the difference between the initial (Co, mg/L) and final concentration (Ce, mg/L) in every flask, as follows:
where qe is the metal uptake capacity (mg/g), V the volume of the metal solution in the flask (L) and M is the dry mass of biosorbent (g).
Results and discussion
FTIR analysis
To investigate the functional groups of FL and metal loaded FL, a FTIR study was carried out and the spectra are shown in Figure 1 (a and b). The FL leaves display a number of absorption peaks, reflecting the complex nature of the FL. A strong and broad peak at 3,348 cm−1 results due to the stretching of the N-H bond of amino groups, which is indicative of bonded hydroxyl group. A change in peak position to 3,336 cm−1 in the spectrum of the metal-loaded FL indicates the binding of cadmium or lead with amino and hydroxyl groups [22–24]. The strong absorption peak at 2,927 cm−1 could be assigned to -CH stretching vibrations of -CH3 and -CH2 functional groups. The peak at 1,570 cm−1 indicates the fingerprint region of CO, C-O and O-H groups, which exists as functional groups of FL. Shifting of this peak to 1,666 cm−1 indicated involvement of these groups in metal binding. The regions between 1,481 and 1,000 cm−1 are the fingerprint region, OH and C-H bending vibration, and C-O stretching vibration absorption bands. The absorption peaks at 1,384 and 1,119 cm−1 could be attributed to the presence of C-O stretching. These peaks were shifted to 1,477 and 1,014 cm−1, respectively. The intense band at 1,014 cm−1 can be assigned to the C-O of alcohols and carboxylic acids. The shift of the peak from 1,084 to 1,014 cm−1 also suggests the involvement of the C-O group in binding Cd(II) [22–24].The shifts in the absorption peaks generally observed indicate the existence of a metal binding process taking place on the surface of the F. carcia leaves.
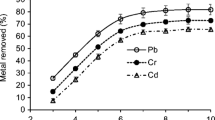
Effect of pH
The effect of pH on the biosorption of Cd(II) and Pb(II) ions onto FL was studied at pH 2 to 8 conditions. The maximum biosorption was observed at pH 5.0 for Pb(II) ions and pH 6.0 for Cd(II) ions, as shown in Figure 2. At lower pH values Cd(II) and Pb(II) removal was inhibited, possibly as a result of the competition between hydrogen and metal ions on the sorption sites, with an apparent preponderance of hydrogen ions, which restricts the approach of metal cations as a consequence of the repulsive force. As the pH increased, the active sites in FL would be exposed, increasing the negative charge density on the FL surface, increasing the attraction of metal ions with positive charge, and allowing the biosorption onto the FL surface. In this study, the Cd(II) and Pb(II) ions at pH 5.0 to 6.0 would be expected to interact more strongly with the negatively charged binding sites in the biosorbent, as shown in Figure 2. The decrease in Cd(II) and Pb(II) biosorption above pH 5.0 to 6.0 was probably due to the precipitation of Cd(II) and Pb(II) ions as cadmium and lead hydroxides, but not due to biosorption. As a result, the optimum pH for cadmium and lead ions biosorption was found as 5.0 and 6.0, respectively; and the other biosorption experiments were performed at this pH value.
Effect of contact time
The rate of biosorption is important for designing batch biosorption experiments. Therefore, the effect of contact time on the biosorption of metals was investigated. The biosorption of Cd(II) and Pb(II) ions increased considerably until the contact time reached 80 min at 30°C. Further increase in contact time did not enhance the biosorption process; so, the optimum contact time was selected as 80 min for further experiments (Figure 3).
Effect of the adsorbent concentration
The adsorbent concentration effect on the percentage removal at equilibrium conditions was investigated. It was observed that the amount of Cd(II) and Pb(II) biosorbed varied with varying adsorbent concentration. The amount of Cd(II) and Pb(II) adsorbed increases with an increase in adsorbent concentration from 0.1 to 0.5 g/0.1L. The percentage of metal removal was increased from 58.44% to 98.58% for an increase in FL concentration from 0.1 to 0.5 g/0.1L at initial concentration of 40 mg/L. The increase in the adsorption of the amount of solute is obvious due to the increasing FL surface area.
Biosorption isotherms
An adsorption isotherm describes the fraction of sorbate molecules that are partitioned between liquid and solid phases in equilibrium. The adsorption of Cd(II) and Pb(II) ions onto FL particles was modeled using two adsorption isotherms: Freundlich and Langmuir isotherms.
Freundlich isotherm
The Freundlich isotherm model is the well-known earliest relationship describing the adsorption process. This model applies to adsorption on heterogeneous surfaces with the interaction between adsorbed molecules, and the application of the Freundlich equation also suggests that sorption energy exponentially decreases on completion of the sorption centers of an adsorbent. This isotherm is an empirical equation and can be employed to describe heterogeneous systems and is expressed as follows in linear form [25]:
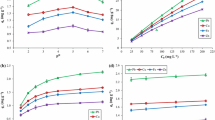
where KF (mg/g)(L/mg)1/n is the Freundlich constant related to the bonding energy; 1/n is the heterogeneity factor and n (L/g) is a measure of the deviation from linearity of adsorption. Freundlich equilibrium constants were determined from the plot of log qe versus log Ce, Figures 4 and 5, on the basis of the linear of Freundlich equation (Equation 1). The n value indicates the degree of nonlinearity between solution concentration and adsorption as follows: if n = 1, then adsorption is linear; if n < 1, then adsorption is a chemical process; if n > 1, then adsorption is a physical process. The n value in Freundlich equation was found to be 2.39 to 3.96 for FL, Table 1. Since n lies between 1 and 10, this indicates the physical biosorption of cadmium(II) and lead(II) onto FL.
Langmuir isotherm
The Langmuir isotherm assumes monolayer adsorption on a uniform surface with a finite number of adsorption sites [26]. Once a site is filled, no further sorption can take place at that site. As such, the surface will eventually reach a saturation point where the maximum adsorption of the surface will be achieved. The linear form of the Langmuir isotherm model is described as
where KL is the Langmuir constant related to the energy of adsorption and qmax is the maximum adsorption capacity (mg/g). Values of Langmuir parameters qmax and KL were calculated from the slope and intercept of the linear plot of Ce/qe versus Ce as shown in Figures 6 and 7. Values of qmax, KL, and regression coefficient R2 are listed in Tables 1 and 2. These values for FL biosorbent indicated that Langmuir theory describes the biosorption phenomena as favorable.
The essential characteristics of the Langmuir isotherm parameters can be used to predict the affinity between the sorbate and sorbent using separation factor or dimensionless equilibrium parameter, RL, expressed as in the following equation:
where KL is the Langmuir constant and Co is the initial concentration of metal ions. The value of separation parameter RL provides important information about the nature of adsorption. The value of RL indicated the type of Langmuir isotherm to be irreversible (RL = 0), favorable (0 < RL < 1), linear (RL = 1), or unfavorable (RL > 1). The RL was found to be 0.21 to 0.67 for concentrations of 10 to 100 mg/L of Cd(II) and Pb(II). They are in the range of 0 to 1 which indicates the favorable biosorption.
Adsorption kinetic
Parameters from two kinetic models, pseudo-first-order and pseudo-second-order, were fit to experimental data to examine the adsorption kinetics of cadmium(II) and Pb(II) uptake by FL.
Pseudo-first-order kinetics
The pseudo-first-order equation of Lagergren [27] is generally expressed as follows:
where q e and q t are the sorption capacities at equilibrium and at time t, respectively (mg/g), and k 1 is the rate constant of pseudo-first-order sorption (1/min). After integration and applying boundary conditions, q t = 0 to qt = qt at t = 0 to t = t, the integrated form of Equation 5 becomes
The equation applicable to experimental results generally differs from a true first-order equation in two ways: the parameter k 1 (qe− q t ) does not represent the number of available sites, and the parameter log qe is an adjustable parameter which is often not found equal to the intercept of a plot of log (qe − q t ) against t, whereas in a true first-order sorption reaction, log qe should be equal to the intercept of log(qe − q t ) against t. In order to fit Equation 5 to the experimental data, the equilibrium sorption capacity qe must be known. In many cases, qe is unknown and as chemisorption tends to become unmeasurably slow, the amount sorbed is still significantly smaller than the equilibrium amount. In most cases in the literature, the pseudo-first-order equation of Lagergren does not fit well for the whole range of contact time and is generally applicable over the initial 20 to 60 min of the sorption process. Furthermore, one has to find some means of extrapolating the experimental data to t = ∞, on treating qe as an adjustable parameter to be determined by trial and error. For this reason, it is therefore necessary to use trial and error to obtain the equilibrium sorption capacity, in order to analyze the pseudo-first-order model kinetics.
The pseudo-first-order rate constant can be obtained from the slope of plot between log (qe − q t ) against time, t. Figure 8 shows the Lagergren pseudo-first-order kinetic plot for the biosorption of Cd(II) and Pb(II) ions onto FL. The pseudo-first-order rate constant values were calculated from the slope of Figure 8. The calculated values and their corresponding linear regression correlation coefficient values are shown in Table 3. The linear regression correlation coefficient values show that this model cannot be applied to predict the adsorption kinetic model.
Pseudo-second-order kinetics
The pseudo-second-order rate expression, which has been applied for analyzing chemisorption kinetics rate, is expressed as
where qe and q t are the sorption capacity at equilibrium and at time t, respectively (mg/g); and k2 is the rate constant of pseudo-second-order sorption, (g/mg/min). For the boundary conditions, q t = 0 to q t = q t at t = 0 to t = t, the integrated form of Equation 7 becomes
Equation 8 can be rearranged to obtain
The linear form of Equation 9
where t is the contact time (min), qe (mg/g) and q t (mg/g) are the amount of the solute adsorbed at equilibrium and at any time, t. If pseudo-second-order kinetics is applicable, the plot of t/q t versus t of Equation 9 should give a linear relationship, from which qe and k can be determined from the slope and intercept of the plot, Figure 9. The pseudo-second-order rate constant k2, the calculated qe value, and the corresponding linear regression correlation coefficient value are given in Table 3. At all initial metal concentrations, the linear regression correlation coefficient R2 values were higher. The higher values confirm that the adsorption data are well represented by pseudo-second-order kinetics.
Comparison of F. carcia leaves with other biosorbents
A comparative study of the maximum biosorption capacity (qmax) of F. carcia leaves with those of some other biosorbents reported in literature is given in Table 4. Differences in qmax are due to the nature and properties of each biosorbent such as surface area and the main functional groups in the structure of the biosorbent. A comparison with other adsorbents indicated a high Cd(II) and Pb(II) biosorption capacity of the F. carcia leaves.
Experimental
All the chemicals used were of analytical reagent (AR) grade purchased from Merck (Germany). All the solutions were prepared with double-distilled water with zero levels of dissolved oxygen. The cadmium(II) and lead(II) stock solutions were prepared from cadmium nitrate [Cd(NO3)2] and lead nitrate [Pb(NO3)2], respectively. F. carcia leaves were washed with distilled water several times to remove dust and soluble materials and then allowed to dry at room temperature. The dried leaves were grounded to a fine powder and sieved to get size fraction <44 μm and then dried in an oven at 60°C for 4h. Cadmium(II) and lead(II) concentrations were determined by AAS600 atomic spectrophotometer (Shimadzu, Japan). pH measurements were made with a WTW pH meter using a combined glass electrode. Fourier transform infrared spectroscopy IR prestige-21 (Shimadzu, Japan) was used to study the functional groups in biosorbent and biosorbent-loaded metal.
Conclusions
The potential of FL for the removal of Cd(II) and Pb(II) ions from aqueous solutions was dependent on biosorption process such as pH, initial metal ion concentrations, biosorbent dose, contact time, and temperature. The equilibrium data have been analyzed using Langmuir and Freundlich isotherms. The characteristic parameters for each isotherm and related correlation coefficients R2 were determined. The Langmuir biosorption isotherms were demonstrated to provide the best correlation for the biosorption of Cd(II) and Pb(II) ions onto FL. The maximum monolayer biosorption capacity of FL was found to be 30.31 mg of Cd(II)/g of FL and 34.36 mg of Pb(II)/g of FL. The kinetic results provided the best correlation of the experimental data of biosorption of Cd(II) and Pb(II) onto FL by pseudo-second-order equation. It can be concluded that since the FL is an easily locally available low-cost adsorbent and has a considerable high biosorption capacity, it may be treated as an alternative adsorbent for treatment of wastewater containing cadmium(II) and lead(II) ions.
References
Horsell M, Spiff AI: Effects of temperature on the sorption of Pb2+ and Cd2+ from aqueous solution by Caladium bicolor (wild cocoyam) biomass. Electron J Biotechnol 2005, 8: 43–50.
Babarinde NAA: Kinetic, equilibrium and thermodynamic of the biosorption of Pb(II), Cd(II) and Cr(III) by neem leaf. J Innovative Res Eng Sci 2011, 2: 291–306.
Chakravarty P, Sarma NS, Sarma H: Biosorption of cadmium(II) from aqueous solution using heartwood powder of Areca catechu . Chem Eng J 2010, 162: 949–955. 10.1016/j.cej.2010.06.048
Sangi MR, Shahmoradi A, Zolgharnein J, Azimi GH, Ghorbandoost M: Removal and recovery of heavy metals from aqueous solution using Ulmus carpinifolia and Fraxinus excelsior tree leaves. J Hazard Mater 2008, 155: 513–522. 10.1016/j.jhazmat.2007.11.110
Pino GH, Souza de Mesquita LM, Torem ML, Pinto GAS: Biosorption of cadmium by green coconut shell powder. Miner Eng 2006, 19: 380–385. 10.1016/j.mineng.2005.12.003
Iqbal M, Saeed A, Zafar SI: FTIR spectrophotometry, kinetic and adsorption isotherms modeling, ion exchange and EDX analysis for understanding the mechanism of Cd2+and Pb2+ removal by mango peel waste. J Hazard Mater 2009, 164: 161–171. 10.1016/j.jhazmat.2008.07.141
Kelly-Vargas K, Cerro-Lopez M, Reyna-Tellez S, Bandala ER, Sanchez Salas JL: Biosorption of heavy metals in polluted water using different waste fruit cortex. Phys Chem Earth 2012, 39: 26–39.
Reddy DHK, Harinath Y, Seshaiah K, Reddy AVR: Biosorption of Pb(II) from aqueous solutions using chemically modified Moringa oleifera tree leaves. Chem Eng J 2010, 162: 626–634. 10.1016/j.cej.2010.06.010
Babarinde NAA, Babalola JO, Sanni SO: Isotherm and thermodynamic studies of the biosorption of Cd(II) from solution by maize leaf. Inter J Phys Sci 2007, 2: 207–211.
Barka N, Abdennouri M, Boussaoud A, El Makhfouk M: Biosorption characteristics of cadmium(II) onto Scolymus hispanicus L. as low-cost natural biosorbent. Desalination 2010, 258: 66–71. 10.1016/j.desal.2010.03.046
Almasi A, Omidi M, Khodadadian M, Khamutian R, Gholivand MB: Lead(II) and cadmium(II) removal from aqueous solution using processed walnut shell: kinetic and equilibrium study. Toxicol Environ Chem 2012, 94: 660–671. 10.1080/02772248.2012.671328
Sousa FW, Sousa MJ, Isadora RN, Oliveira IRN, Oliveira AG, Cavalcante RM, Fechine PBA, Neto VOS, de Keukeleire D, Nascimento RF: Evaluation of a low-cost adsorbent for removal of toxic metal ions from wastewater of an electroplating factory. J Environ Manag 2009, 90: 3340–3344. 10.1016/j.jenvman.2009.05.016
El-Ashtoukhy ESZ, Amin NK, Abdelwahab O: Removal of lead(II) and copper(II) from aqueous solution using pomegranate peel as a new adsorbent. Desalination 2008, 223: 162–173. 10.1016/j.desal.2007.01.206
Kazemipour M, Ansari M, Tajrobehkar S, Majdz M, Kermani HR: Removal of lead, cadmium, zinc and copper from industrial carbon developed from walnut, hazelnut, almond, pistachio and apricot stone. J Hazard Mater 2008, 150: 322–327. 10.1016/j.jhazmat.2007.04.118
Mitic-Stojanovic DL, Zarubica A, Purenovic M, Bojic D, Andjelkovic T, Bojic AL: Biosorptive removal of Pb2+, Cd2+ and Zn2+ ions from water by Iagenaria vulgaris shell. Water SA 2011, 37: 303–312.
Martinez M, Miralles N, Hidalgo S, Foil N, VillaescusaI PJ: Removal of lead(II) and cadmium(II) from aqueous solution by grape stalk waste. J Hazard Mater 2006, 133: 203–211. 10.1016/j.jhazmat.2005.10.030
Asrari E, Tavallali H, Hagshenas M: Removal of Zn(II) and Pb(II) ions using rice husk in food industrial wastewater. J Appl Sci Manag 2010, 14: 159–162.
Nagpal UMK, Bankar AV, Pawar NJ, Kapadnis P, Zinjarde SS: Equilibrium and kinetic studies on biosorption of heavy metals by leaf powder of paper mulberry ( Broussonetia papyrifera ). Water Air Soil Poll 2011, 215: 177–188. 10.1007/s11270-010-0468-z
Abdel-Ghani NT, Hefny MM, El-Chaghaby GA: Removal of metal ions from synthetic wastewater by adsorption onto Eucalyptus camaldulenis tree leaves. J Chil Chem Soc 2008, 53: 1585–1587.
Goyal P, Sharma P, Srivastava S, Srivastava MM: Saraca indica leaf powder for decontamination of Pb: removal, recovery, adsorbent characterization and equilibrium modeling. Int J Environ Technol 2008, 5: 27–34.
Blazquez G, Martin-Lara MA, Tenorio G, Calero M: Batch biosorption of lead(II) from aqueous solution by olive tree pruning waste: equilibrium, kinetics and thermodynamic study. Chem Eng J 2011, 168: 170–177. 10.1016/j.cej.2010.12.059
Aharoni A, Ungarish M: Kinetics of activated chemisorption part 2. Theoretical models. J Chem Soc Faraday Trans 1977, 73: 456–464.
Boparai HK, Joseph M, O'Carroll DM: Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J Hazard Mater 2011, 186: 458–465. 10.1016/j.jhazmat.2010.11.029
Ho YS: Review of second order models for adsorption systems. J Hazard Mater 2006, 136: 681–689. 10.1016/j.jhazmat.2005.12.043
Freundlich H, Hellen W: The adsorption of cis - and trans -azobenzen. J Amer Chem Soc 1993, 61: 2–28.
Langmuir I: The adsorption of gases on plane surfaces of glass, mica and platinum. J Amer Chem Soc 1918, 40: 1361–1403. 10.1021/ja02242a004
Lagergren S: About the theory of so-called adsorption of soluble substances. K. Sven Vetenskapsakad Handl 1898, 24: 1–39.
Acknowledgment
Authors are thankful to Royal Scientific Society, Amman, Jordan and Baghdad University, Baghdad, Iraq for providing the necessary facilities to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AMA participated in the design of the study, and carried out the experimental work and drafted the manuscript. AHA studied kinetics of the biosorption process. AMF participated in the design of the study and performed the analysis of the samples. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Farhan, A.M., Al-Dujaili, A.H. & Awwad, A.M. Equilibrium and kinetic studies of cadmium(II) and lead(II) ions biosorption onto Ficus carcia leaves. Int J Ind Chem 4, 24 (2013). https://doi.org/10.1186/2228-5547-4-24
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5547-4-24