Abstract
Glycation is a general spontaneous process in proteins which has significant impact on their physical and functional properties. These changes in protein properties could be related to several pathological consequences such as cataract, arteriosclerosis and Alzheimer’s disease. Among the proteins, glycation of Human serum albumin (HSA) is of special interest. Human serum albumin is the most abundant protein in the plasma and because of its high sensitivity for glycation, undergoes structural and functional changes due to binding of reducing sugars in vitro. The glycation process occurs by plasma glucose in vivo which has great impacts on the three dimensional structure of protein. These changes are efficient and stable enough which makes the protein to be considered as a new special disease marker instead of HbA1C for diabetes. In some cases, glycated albumin was used as an alternative marker for glycemic control. Glycated albumin reacts with glucose ten times more rapidly than HbA1C and has shorter half-life which makes it more reliable for indicating glycemic states. In this review, glycation of Human Serum Albumin has been overviewed, starting from overall concepts of glycation, followed by some Examples of pathological consequences of protein glycation. The BSA aggregation was reviewed in terms of structural and biological impacts of glycation on the protein followed by reporting documents which indicate possibility of glycated albumin to be used as specific marker for diabetes. Finally, some of the studies related to the models of glycated albumin have been briefly described, with an emphasis on In vitro studies. It is interesting to note the relationship found between in vitro glycation experiments and the propensity of proteins to form amyloid structures, a point that could be further explored as to its significance in hyperglycemic states.
Similar content being viewed by others
Introduction
Albumin is one of longest known proteins of plasma [1]. Normal concentration of albumin is 35–50 g/l, which makes it the most abundant protein in plasma with a wide variety of physiological functions. Human albumin presents 50% of the normal individual’s plasma protein [2]. The protein is organized into three domains, I, II and III, each subdivided into two subdomains, A and B [3], with 17 intramolecular disulfide bonds which makes it suitable for a wide variety of modifications including response to pH and other biophysical compounds [4]. Due to its low molecular weight (67 kDa), albumin contributes in osmotic pressure maintenance of plasma, compared with other plasma globulins [5], and also because of its weak isoelectric point, the protein has a global negative charge at physiological pH [6]. Albumin structure allows protein to bind and transport diverse metabolites such as metal ions, fatty acids, bilirubin and drugs [7, 8]. Indeed, conjugation of drugs with this protein with long half-life, improved their pharmacokinetic properties [9].
There are three main binding sites on the protein; two of them (site I and site II) are located on subdomains IIA and IIIA, respectively [10] and have been found to bind specifically aromatic and heterocyclic ligands [3]. Flexibility of human serum albumin (HSA) enables interaction of the protein with numerous compounds, by contrast, site II, which is less flexible, has not same property. As examples, paracetamol, a commonly used analgesic drug, binds to residues located in the subdomain IIIA [11, 12] and other metabolites such as fatty acids bind to other locations, whose seven binding sites are localized in subdomains IB, IIIA, and IIIB [13]. In addition, some residues, such as cysteine, lysine, serine and arginine, have found to covalently bind to many drugs [14].
When protein modification induced by physiological or pathological changes occurs, an alteration of the native conformation and efficiency of these binding sites can be expected [15]. Advanced Glycation End Products (AGE), which could bind to plasma proteins, were considered as a novel class of uremic toxins [16], and besides ceruloplasmin and other plasma compounds such as ascorbic acid [17], the role of albumin has been highlighted as an antioxidant, having a part in keeping the body safe in cases of oxidative stress [18] which is due to availability of several residues to work as antioxidant [19]. Cys-34 is one of the powerful residues involved in disulfide bond and in a healthy person 70-80% of albumin Cys-34 is in its reduced form and is able to scavenge hydroxyl radicals [20] when the protein is in native conformation [21].
Another amino acid, methionine, is involved in the antioxidant activity of protein. The more exposed methionine residues are presented to oxidation, could serve as ROS (reactive oxygen species) scavenging system to protect proteins from oxidation [22]. Other main binding sites could be mentioned. For instance, carbohydrates could bind to albumin via three main sites (Lys-351, Lys-475 and Arg-117), and as so, albumin may protect other proteins from glycation in the initial stages of diabetes [23].
Many studies have been done on bovine serum albumin (BSA) which has high (76%) sequence homology to human serum albumin (HSA). BSA, with 583 amino acids and molecular weight of 66.28 [24], has an ellipsoidal shape and like HSA, includes three homologous domains (I, II and III), which are connected together through helical extensions. Similar to HSA, it contains 17 disulfide bridges which make the structure stable in neutral pH and room temperature. The free thiol group of Cys-34 is also present in BSA. It has been found to have a relevant role in thermal aggregation of the protein [25]. BSA shows several conformational isomers at different pH values; lots of these structural changes are due to breakage of ionic bonds. The three dimensional structure of the protein changes from nature (N) form, hold at pH 4–8, to the unfolded state at pH 3, where a decrease of helical structure is observed [26].
As mentioned, BSA and HSA have close homology in primary structure; a main difference, important in structural studies, is believed to be due to the presence of two tryptophan residues (Trp-131, and Trp-214) in BSA, while one (Trp-214) is present in HSA [27, 28]. The tryptophan 214 localized at domain II of HSA is suggested to have a role in its amyloid fibril formation [29].
In this review, we provide an outline on human serum albumin and its glycation in diabetic patients, which is recently suggested to have high potential as a possible diagnostic marker alongside with the well-known HbA1C. Some pathological effects of glycation are also mentioned. Finally, an introduction to in vitro models of albumin glycation, as valuable tools in the study of this modified protein is also included.
Glycation of proteins
Glycation, i.e., the non-enzymatic addition of carbohydrate moieties to protein reactive residues, has been the subject of many studies over the last decade. The process is fastened in diabetic states, which provided the preliminary ground to assess the relationship between elevated levels of glycated hemoglobin A1C and this disease [30]. In vivo glycation has been described in proteins such as the lens crystallins [31], collagen, ferritin, apolipoprotein [32], and serum albumin [33]. In addition to glucose, sugars such as galactose [34], fructose, ribose [35] sialic acid [34], mannose [36], glucose 6-phosphate [37], glyceraldehydes [38], and fucose [39] have been used in vitro as glycating agents, sometimes to fasten the process.
These sugars undergo maillard reaction corresponding to a condensation between a carbonyl compound of reducing sugar and a free amino group of specific residues such as lysine or arginine [40] and besides the solubility of the end-stage products, the reaction intermediates may be dark brown aggregates [41]. Early glycation leads to the formation of Schiff's bases and Amadori products and Further oxidation produces advanced glycated end products (AGE) [42]. During the production of fluorescent AGEs products, proteins undergo both glycation and oxidation. As the early-stage of the reaction includes interaction of reducing sugars, such as glucose, with free amino groups of lysine and arginine residues, recent studies have demonstrated that the glycation process is facilitated readily by the presence of a histidine residue near the lysine [43]. The late-stage of reaction is an irreversible cascade of reactions involving dehydration, hydrolysis, etc., leading to the formation of AGE. AGEs products are considered to be a marker of various diseases, such as arteriosclerosis, renal failure, Alzheimer disease, or diabetes, but they also increase during normal aging [44, 45].
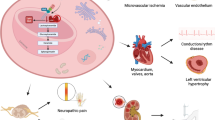
AGE products exhibit a wide range of chemical structures and thereby have different biological [46, 47] properties (Figure 1). Major chemically characterized AGEs are N-(carboxymethyl)lysine (CML) [48], pentosidine [49], pyrraline [50], and imidazolone [51].
Protein cross-linking by AGEs results in the formation of detergent-insoluble aggregates [52, 53]. Such aggregates may have interference with protein degeneration and other metabolic disorders during the diabetes or other in vivo glycating conditions in the body such as pregnancy.
Examples of pathological consequences of protein glycation
Most of the studies on glycation contributed to diseases have been primarily focused on diabetes and diabetes-related complications [54, 55]. However, resulting damages are, of course, not limited to diabetic patients and even at normal glucose levels some degree of glycation and resulting damage occurs. Such damages are observed in various diseases such as cataract, arteriosclerosis, and neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease [56], Creutzfeld-Jacob disease [40] and amyotrophic lateral sclerosis (ALS) [33].
In general, these random damage-induced posttranslational modifications of proteins are basic causatives of protein degradation [57–60]. However, this oxidative cross linking of proteins is accompanied by a decreased proteolytic susceptibility and therefore accumulation of these oxidized proteins [61]. For example Friguet et al. [62] reported a decreased proteolytic susceptibility of N-carboxymethyl lysine- glycated glucose-6-phosphate dehydrogenase. Besides that, the degradation of AGE modified proteins might be a complex process, including various uptake mechanisms, several proteases or peptidases, and perhaps other additional hydrolases, involving in lipid modifications [63].
Cataract
Cataract is one of the major causes of impaired vision which caused blindness in patients with long time elevated glucose levels. Indeed diabetes has been thought to increase the risk of its development. At least two mechanisms have been mentioned for development of cataract in diabetic subjects: firstly, the aldose reductase-osmotic mechanism and, secondly, the glycation hypothesis. As a matter of fact, the glycation hypothesis may be more compatible with the slow progress of cataract in diabetic patients.
A lot of reports, confirms glycation of lens crystallin in the case of diabetic cataract [64–66]. Liang et al. (1986) reported increased glycation of α-crystaliin compared to β and γ-crystallins. The data of Stevens et al. (1978) showed that glycation of γ-crystallin was higher than α and β-crystallins in diabetic rats and also Chiou et al. (1981) observed that γ -crystallin glycation was equal to or higher than that of α and β-crystallins and other studies showing glycation of different crystallins. In rat lens, the soluble protein fraction diminishes considerably with aging and diabetes and the proportion of different crystallins depleted from the soluble fraction may also vary [64]. It is believed that glycation causes lens crystallins to aggregate into high molecular weight (HMW) aggregates [67].
Arteriosclerosis
Arteriosclerosis in its wide sense, including athero- and arteriosclerosis occurs more frequently in diabetics than in non diabetic individuals [68]. Diabetes leads to arteriosclerotic diseases such as coronary artery occlusion and cerebrovascular. In addition, hyperglycemia causes aortic intramural accumulation of sorbitol, which leads to increased osmolarity and thus to intramural water retention and decreased tissue oxygenation [69]. Glycation of low density lipoprotein and collagen in the vascular wall are also considered as effective factors of arteriosclerosis [70]. Elastin is another important protein component of elastic fibers of arterial media with a long biological half-life which glycation causes macroangiopathy in diabetic individuals [71]. Actually the elastin content per unit volume of thoracic aorta decreases more rapidly in diabetics than in normal control individuals. Indeed the content of collagen and glycosaminoglycan increases in parallel with calcium deposition and therefore accumulation of degenerated elastin in diabetics.
Alzheimer’s disease
Alzheimer’s disease (AD), the leading cause of senile dementia, is another glycated protein related disease which is characterized by formation of senile plaques in brain. A wide variety of studies have demonstrated the increased accumulation of AGEs in AD brain [72–74]. AGE modifications and oxidative-stress mechanisms can both impair the neuronal proteins and result in plaques formation [75]. Amount of glycation in cerebrospinal fluid (CSF) is another cause of ageing and AD. Indeed, glycation of CSF proteins which is reported in the CSF of AD patients is suggested to be a triggering cause of AD. It is proposed that the high level of glycation in AD may be accompanied by numerous neuropathological consequences due to damaged proteins [72].
Glycation of albumin
Human Serum Albumin is very sensitive to glycation. As mentioned before, the slow, non-enzymatic Maillard reaction initially involves the attachment of glucose or other carbohydrate compounds such as galactose and fructose, to the free amine groups of albumin [35]. The glycation efficiency depends on the nature and the anomerization of the carbohydrate involved in the process. As an example, in comparison with glucose, ribose induces a faster glycation process with albumin and forms amyloid-like products [76]. In vivo studies demonstrated that the proportion of glycated albumin in healthy persons is in the range of 1- 10% [3], compared with diabetic individuals in whom this may increase two- to three fold [77].
Impact of glycation on albumin structure
In vitro or in vivo studies that have been performed on structural properties of glycated albumin include both human and bovine albumin. Comparing sequences of human and bovine albumin reveals a homology close to 80% [78]. Observed differences are mainly proposed to be structurally conservative, e.g. hydrophobic amino acids are replaced by other hydrophobic amino acids and therefore the main three dimensional structures is the same. The glycation of albumin induces several structural modifications, including an increase in total molecular weight of the protein due to the glycation [79]. Non-enzymatic glycosylation of albumin occurs at multiple residues such as arginine, lysine and also cysteine. So far, a lot of studies focused on the main sites modified by glycation for serum albumin have been done in vivo. Lysine, arginine and cysteine residues are subjected to glycation mostly because of their high nucleophile properties. Lysine-525 (Figure 2) is considered to be the predominant site of the of human serum albumin in vivo glycation which constitutes 30% of the overall glycation of the protein by glucose [80]. Alongside with Lys-525 which appears to be the most reactive glycation site, in native conformation, other lysine residues, such as 199, 276, 281,378, 439, and 545 have been found to have lower participation than Lys-525 in overall glycation [81, 82]. For example, Lys-199 accounts for only 5% of total glycation. In addition, other residues of less importance have also been identified [80] which are located in the vicinity of known drug binding sites in HSA [82]. These charged amino acid residues at physiological pH may have an acid-based role for Amadori rearrangement. The Lys-199 and Lys-281 are close to disulfide bonds, which present a positively charged amino group, close to these sites and higher accessibility of some amine residues depends on the tertiary structure conformation of albumin.
Regardless of residues located at the amino terminal of the albumin, evidence for the reactiviy of other lysine residues, such as Lys-12, Lys-233, Lys-317, or Lys-351, is less certain [83]. This hypothesis is established by the fact that in vivo copper-albumin complex cannot be glycated. The extent of glycation depends on the glycemic status and also the half-life of the protein.
Other researches propose lysine-524 (equivalent to Lys-525 of HSA) as the major glycation site in BSA [84] and Lys-275 (equivalent to Lys-276 of HSA), Lys-232 (equivalent to Lys-233 of HSA) and Lys-396 are susceptible to be glycated [84].
Although less than lysine residues (24 for 59 lysine residues), arginine residues can also be involved in glycative modification of HSA (Figure 2). Arg-410 appears to be the predominant glycation site [85] and other involving but not major residues such as Arg-114, Arg-160, Arg-186, Arg-218 and Arg-428, have also been identified [82].
Finally, the thiol group of cysteine residues is powerful nucleophile, which can also be glycated in vitro by methylglyoxal to give rise to AGEs such as S-carboxymethyl-cysteine (CMC) [86]. CMC occurrence in plasma from diabetic patients, suggest the involvement of Cys-34 in the glycation process [87].
Glycoxidation of albumin is accompanied by structural modifications in both in vitro and in vivo glycation process. The interrelationship between glycation and amyloid formation is interesting in this regard, as criteria for glycation completion was the production of the amyloid nanofibril after 20 weeks of incubation with glucose. This has caused a decrease in hydrophobicity of the protein [88] due to the transition in albumin from a helical to a beta-sheet structure. Glycation induced aggregation is not necessarily associated with secondary structure modification. In addition, the glycation process could result in an overall stabilization in both tertiary and secondary structure of the protein which increases the protein stability and improves the protein life time [33].
Biological impact
Glycated albumin has an important clinical implication, since it is involved in the damages associated with the diabetes mellitus, such as retinopathy, nephropathy, neuropathy and coronary artery disease [89]. In most studies, the deleterious effects of glycated albumin have been highlighted for instance the physiopathological correlation between glycated albumin and diabetic renal deficiency and microangiopathy [90] and also diabetic retinopathy [91]. Several in vitro studies have shown the implication of glycated albumin in platelet activation and aggregation [92]. Beside platelets, the lowering aggregation effects of glycated albumin have also been demonstrated in erythrocytes [93].
Another pathogenic implication of glycated albumin can also be observed in glucose metabolism of both skeletal muscle and adipocyte cells [94]. It has been found that in mouse, adipocyte cell lines, albumin-derived species triggers generation of intracellular ROS leading to an inhibition of glucose uptake which also cause attenuation of adipocyte insulin sensitivity and microangiopathy [90]. More recently, proteins such as nucleophosmin in monocyte like cell membrane and Calnexin, a transmembrane protein, were reported to serve as receptors for Amadori-glycated albumin [95].
Glycated albumin, a possible specific marker for diabetes
The glycation process of proteins occurs in a higher amount in diabetic patients compared to non-diabetic individuals. This is strongly involved in the development and progression of chronic diabetic complications. The two main clinical parameters of diabetes are the glycated hemoglobin (HbA1C) and the blood glucose levels [96]. The first parameter is as long term indicator of diabetes, because of long term half life of erythrocytes(about 120 days) and could reflects the glycemic control state over the past 2 months, But the measurement of glucose is a short-term indicator and reflects diabetic status over a 24 h period. However, several studies have reported that in some case, HbA1C values should be considered cautiously. As a matter of fact, glycated hemoglobin levels have invalid correlation to blood glucose levels in patients with hemolytic anemia, or those having hemodialysis or iron deficiency [97].
Thus in numerous case such as hemolytic or renal anemia and liver cirrhosis, HbA1C gives incorrect values and is not suitable marker as a control [98]. Glycated albumin, because of its shorter half-life (21 days) compared with hemoglobin, could be used as a shorter-term glycemic control for diabetes. The glycated albumin level could not to be easily altered by abnormal hemoglobin metabolism [99]. This advantage of glycated albumin is based on two facts. First, the amount of in vivo non-enzymatic glycation of albumin is approximately 9 times more than HbA1C [80]. Secondly, albumin glycation reaction occurs ten times more quickly than hemoglobin glycation [80] so, the glycation phenomenon in plasmatic protein occurs more easily than hemoglobin, which all make the glycated albumin a good additional marker for evaluating glycemic control in type 1 and 2 diabetes [100].
In some studies glycated albumin is suggested as an alternative marker for glycemic control in many diabetes complications, including nephropathy [101], retinopathy [102] and Alzheimer’s disease [72] and also in the case of hemodialysis patients [103] or gestational diabetes [97]. All these data support the possibility use of glycated albumin in the detection of short-term changes in glycemic controls. Glycated albumin levels, determined in different in vivo studies for different pathologies associated to diabetes mellitus, could be consulted in [33].
It should be mentioned that in some cases such as thyroid dysfunction, nephrotic syndrome or liver cirrhosis which the amounts of albumin is affected, and so, glycated albumin level is not a suitable indicator [104]. Similarly, glycated albumin could be influenced by other conditions, such as body mass index (BMI) [105] or the age of diabetic patients [106]. Therefore a combined detection of HbA1C and glycated albumin may improve the efficacy of diagnosis and improvement of a novel therapeutic potential [107].
In vitro models of glycated albumin
As highlighted in the previous sections, studies on the glycation process of Human Serum Albumin (HSA) is of special interest, due to its clinical significance. This final section intends to introduce in vitro models of albumin glycation, as a mean to study this phenomenon under controlled conditions. The significant impact of glycation on the protein structure could be observed more conveniently by in vitro techniques such as the use of heat and pressure [108]. HSA has approximately 58 lysine residues [80]. As mentioned previously, during the glycation process, different sugars and sugar phosphates could undergo traditional Maillard reactions leading formation of various products [109]. Syrový et al. glycated albumin (5 mg/ml) using Hepes (4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid) buffer and carbohydrates including glucose, fructose, galactose, ribose and glyceraldehydes for 20 days at 37°C. They showed that the incubation of albumin was occurring more efficiently in presence of ribose, glyceraldehydes and galactose respectively [110]. Another research was done by Schmitt et al. to study modifications taking place during glycation of 4 mM HSA in different glucose concentrations (5–500 mM) and showed that there is a linear relationship between these two parameters. They found that the percentage of reacted lysines was increasing rapidly up to 200 mM glucose [111]. GhoshMoulick et al. compared the glycation process of albumin and two other proteins, namely hemoglobin and lysozyme, and demonstrated that the ability of albumin glycation is positioned between two others [112]. Sattarahmady et al. compared the ability of glucose, fructose, and ribose in glycation of HSA and generation of amyloid structures and demonstrated that the prolonged exposure of HSA has a detergent-like effect on HSA structure and leads to partial unfolding and hydrophobic surfaces exposure, and finally amyloid formation [35]. In a previous study, they had monitored changes occurring in the three-dimensional structure of the protein, and the number of basic residues modified with glucose [88]. In order to study in vitro formation of AGE-HSA, protein concentration of 1–10 mg/ml have been used in phosphate buffer to be incubated with glucose, fructose, and ribose or other reducing sugars (generally at 500 mM) at 37°C in the dark [35, 110, 113, 114]. After 2, 4, or 20 weeks of incubation, the glycation process could be observed as browning soluble materials. Recently, S. Barnaby et al. have made a comparison of modification sites formed on human serum albumin at various stages of glycation. It was found that the glycation pattern of HSA strongly depends on total amounts of glycation. Many modifications sites including K199, K281, and the N terminus, in addition to lysines 199 and 281, as well as arginine 428 were found in the tested samples. Lysine residues 93, 276, 286, 414, 439, and 524/525, as well as the N-terminus and arginines 98, 197, and 521, were also found to be modified at various degrees of HSA glycation [113].
Glycated HSA could forms micelle-like aggregates upon prolonged glycation. In these cases, glycation of HSA resulted in physicochemical changes including alterations in protein conformation, molecular weight, and pI and all these changes were in the direction of transition from a helical to a β-sheet structure and formation of nanofibrillar amyloid.
Although in all cases mentioned before, using glucose could be more compatible to what happens in the body, in some cases the glycation process is faster by the use of other reducing sugars, while some results could still be interpreted as relevant to the natural conditions. Similarly, using buffers more similar to the body conditions could present more reliable results for the glycation process.
Conclusion
This review has outlined the glycation reaction of proteins including a discussion on the glycation process itself, and particularly with regard to albumin glycation. We give an overview of the role of glycation in the physiopathology of different diseases and specially highlight the properties of glycated albumin. The new aspect of glycated albumin being used as a disease marker, compared with HbA1C is also mentioned. Finally, an introduction to some in vitro models of glycated albumin is made. It is interesting to note the relationship found between in vitro glycation experiments and the propensity of proteins to form amyloid structures, a point that could be further explored as to its significance in hyperglycemic states.
References
Rondeau P, Navarra G, Militello V, Bourdon E: On the Aggregation of Albumin: Influences of the Protein Glycation. In Protein Aggregation. Edited by: Douglas A. Stein Nova Science Publishers, Inc; 2010.
Evans T: Review article: albumin as a drug—biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther 2002, 16: 6–11.
Peters T: ScienceDirect: All about albumin: biochemistry, genetics, and medical applications. CA: Academic Press San Diego; 1996.
Foster J, Rosenoer V, Oratz M: Albumin structure, function and uses. Edited by: Rosenoer VM, Oratz M, Rothschild MA. Oxford: Pergamon; 1977:53–84.
Scatchard G, Batchelder A, Brown A: Chemical, clinical, and immunological studies on the products of human plasma fractionation. VI. The osmotic pressure of plasma and of serum albumin. J Clin Invest 1944, 23: 458.
Figge J, Rossing T, Fencl V: The role of serum proteins in acid–base equilibria. J Lab Clin Med 1991, 117: 453.
Waldmann T, Rosenoer V, Oratz M, Rothschild M: Albumin Structure, Function and Uses. New York: Pergamon Press; 1977.
Wood M: Plasma drug binding: implications for anesthesiologists. Anesth Analg 1986, 65: 786.
Kratz F: Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release 2008, 132: 171–183.
Sudlow G, Birkett D, Wade D: Further characterization of specific drug binding sites on human serum albumin. Mol Pharmacol 1976, 12: 1052.
Weber G: Energetics of ligand binding to proteins. Adv Protein Chem 1975, 29: 68.
Daneshgar P, Moosavi-Movahedi AA, Norouzi P, Ganjali MR, Madadkar-Sobhani A, Saboury AA: Molecular interaction of human serum albumin with paracetamol: Spectroscopic and molecular modeling studies. Int J Biol Macromol 2009, 45: 129–134.
Petitpas I, Bhattacharya AA, Twine S, East M, Curry S: Crystal structure analysis of warfarin binding to human serum albumin. J Biol Chem 2001, 276: 22804.
Qiu Y, Burlingame A, Benet L: Mechanisms for covalent binding of benoxaprofen glucuronide to human serum albumin. Drug Metab Dispos 1998, 26: 246–256.
Oettl K, Stauber R: Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br J Pharmacol 2007, 151: 580–590.
Thornalley PJ, Argirova M, Ahmed N, Mann VM, Argirov O, Dawnay A: Mass spectrometric monitoring of albumin in uremia. Kidney Int 2000, 58: 2228–2234.
Halliwell B, Gutteridge J: The antioxidants of human extracellular fluids. Arch Biochem Biophys 1990, 280: 1–8.
Bourdon E, Blache D: The importance of proteins in defense against oxidation. Antioxid Redox Signal 2001, 3: 293–311.
Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E: The antioxidant properties of serum albumin. FEBS letters 2008, 582: 1783–1787.
Carballal S, Alvarez B, Turell L, Botti H, Freeman B, Radi R: Sulfenic acid in human serum albumin. Amino Acids 2007, 32: 543–551.
Lee H, Kim IH: Thioredoxin-linked lipid hydroperoxide peroxidase activity of human serum albumin in the presence of palmitoyl coenzyme A. Free Radic Biol Med 2001, 30: 327–333.
Levine RL, Berlett BS, Moskovitz J, Mosoni L, Stadtman ER: Methionine residues may protect proteins from critical oxidative damage. Mech Ageing Dev 1999, 107: 323–332.
Bhonsle HS, Singh SK, Srivastava G, Boppana R, Kulkarni MJ: Albumin competitively inhibits glycation of less abundant proteins. Protein Pept Lett 2008, 15: 663–667.
Gelamo E, Tabak M: Spectroscopic studies on the interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants. Spectrochim Acta A Mol Biomol Spectrosc 2000, 56: 2255–2271.
Militello V, Vetri V, Leone M: Conformational changes involved in thermal aggregation processes of bovine serum albumin. Biophys Chem 2003, 105: 133–141.
Arasteh A, Habibi Rezaei M, Ebrahim Habibi A, Moosavi Movahedi Ali A: Bovine serum albumin aggregation: An optimizing approach. Clin Biochem 2011, 44: S136-S137.
Brewer SH, Glomm WR, Johnson MC, Magne K, Franzen S: Probing BSA binding to citrate-coated gold nanoparticles and surfaces. Langmuir 2005, 21: 9303–9307.
Taboada P, Gutiérrez-Pichel M, Mosquera V: Effects of the molecular structure of two amphiphilic antidepressant drugs on the formation of complexes with human serum albumin. Biomacromolecules 2004, 5: 1116–1123.
Dockal M, Carter DC, Rüker F: Conformational transitions of the three recombinant domains of human serum albumin depending on pH. J Biol Chem 2000, 275: 3042–3050.
Bouma B, Kroon-Batenburg LMJ, Wu YP, Brünjes B, Posthuma G, Kranenburg O, de Groot PG, Voest EE, Gebbink MFBG: Glycation induces formation of amyloid cross-β structure in albumin. J Biol Chem 2003, 278: 41810.
Gul A, Rahman MA, Salim A, Simjee SU: Advanced glycation end products in senile diabetic and nondiabetic patients with cataract. J Diabetes Complications 2009, 23: 343–348.
Tomizawa H, Yamazaki M, Kunika K, Itakura M, Yamashita K: Association of elastin glycation and calcium deposit in diabetic rat aorta. Diabetes Res Clin Pract 1993, 19: 1–8.
Rondeau P, Bourdon E: The glycation of albumin: Structural and functional impacts. Biochimie 2011, 93: 645–658.
Suarez G, Rajaram R, Oronsky A, Gawinowicz M: Nonenzymatic glycation of bovine serum albumin by fructose (fructation). Comparison with the Maillard reaction initiated by glucose. J Biol Chem 1989, 264: 3674.
Sattarahmady N, Moosavi-Movahedi AA, Habibi-Rezaei M, Ahmadian S, Saboury AA, Heli H, Sheibani N: Detergency effects of nanofibrillar amyloid formation on glycation of human serum albumin. Carbohydr Res 2008, 343: 2229–2234.
Davis LJ, Hakim G, Rossi CA: Kinetics of the glycation of bovine serum albumin by mannose and fucose in vitro . Biochem Biophys Res Commun 1989, 160: 362–366.
Avigad G, Kniep A, Bailin G: Reaction of rabbit skeletal myosin with D‒glucose 6‒phosphate. IUBMB Life 1996, 40: 273–284.
Choei H, Sasaki N, Takeuchi M, Yoshida T, Ukai W, Yamagishi S, Kikuchi S, Saito T: Glyceraldehyde-derived advanced glycation end products in Alzheimer’s disease. Acta Neuropathol 2004, 108: 189–193.
Ravelojaona V, Robert AM, Robert L: Expression of senescence-associated β-galactosidase (SA-β-Gal) by human skin fibroblasts, effect of advanced glycation end-products and fucose or rhamnose-rich polysaccharides. Arch Gerontol Geriatr 2009, 48: 151–154.
Rondeau P, Navarra G, Cacciabaudo F, Leone M, Bourdon E, Militello V: Thermal aggregation of glycated bovine serum albumin. Biochimica et Biophysica Acta (BBA)-Proteins & Proteomics 2010, 1804: 789–798.
Zarina S, Zhao HR, Abraham E: Advanced glycation end products in human senile and diabetic cataractous lenses. Mol Cell Biochem 2000, 210: 29–34.
Chetyrkin S, Mathis M, Ham A, Hachey D, Hudson B, Voziyan P: Propagation of protein glycation damage involves modification of tryptophan residues via reactive oxygen species: inhibition by pyridoxamine. Free Radic Biol Med 2008, 44: 1276–1285.
Monnier V, Sell D, Nagaraj R, Miyata S, Grandhee S, Odetti P, Ibrahim S: Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremia. Diabetes 1992, 41: 36.
Vlassara H: Advanced glycation in health and disease: role of the modern environment. Ann N Y Acad Sci 2005, 1043: 452–460.
Ulrich P, Cerami A: Protein glycation, diabetes, and aging. Recent progress in hormone research 2001, 56: 1–22.
Kikuchi S, Shinpo K, Ogata A, Tsuji S, Takeuchi M, Makita Z, Tashiro K: Detection of Nepsilon-(carboxymethyl) lysine (CML) and non-CML advanced glycation end-products in the anterior horn of amyotrophic lateral sclerosis spinal cord. Amyotroph Lateral Scler 2002, 3: 63–68.
Vlassara H, Bucala R, Striker L: Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest 1994, 70: 138.
Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR: The advanced glycation end product, N-(carboxymethyl) lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem 1996, 271: 9982.
Dyer D, Blackledge J, Thorpe S, Baynes J: Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo . J Biol Chem 1991, 266: 11654.
Nakayama M, Kawaguchi Y, Yamada K, Hasegawa T, Takazoe K, Katoh N, Hayakawa H, Osaka N, Yamamoto H, Ogawa A: Immunohistochemical detection of advanced glycosylation end-products in the peritoneum and its possible pathophysiological role in CAPD. Kidney Int 1997, 51: 182–186.
Niwa T, Katsuzaki T, Ishizaki Y, Hayase F, Miyazaki T, Uematsu T, Tatemichi N, Takei Y: Imidazolone, a novel advanced glycation end product, is present at high levels in kidneys of rats with streptozotocin-induced diabetes. FEBS letters 1997, 407: 297–302.
Kikuchi S, Shinpo K, Takeuchi M, Yamagishi S, Makita Z, Sasaki N, Tashiro K: Glycation–a sweet tempter for neuronal death. Brain Res Rev 2003, 41: 306–323.
Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G: Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci 1994, 91: 5710.
Myint T, Hoshi S, Ookawara T, Miyazawa N, Suzuki K, Taniguchi N: Immunological detection of glycated proteins in normal and streptozotocin-induced diabetic rats using anti hexitol-lysine IgG. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1995, 1272: 73–79.
Ryle C, Leow C, Donaghy M: Nonenzymatic glycation of peripheral and central nervous system proteins in experimental diabetes mellitus. Muscle & Nerve 1997, 20: 577–584.
Castellani R, Smith M, Richey G, Perry G: Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res 1996, 737: 195–200.
Chondrogianni N, Stratford FLL, Trougakos IP, Friguet B, Rivett AJ, Gonos ES: Central role of the proteasome in senescence and survival of human fibroblasts. J Biol Chem 2003, 278: 28026–28037.
Mary J, Vougier S, Picot CR, Perichon M, Petropoulos I, Friguet B: Enzymatic reactions involved in the repair of oxidized proteins. Exp Gerontol 2004, 39: 1117–1123.
Chen Q, Ding Q, Thorpe J, Dohmen RJ, Keller JN: RNA interference toward UMP1 induces proteasome inhibition in Saccharomyces cerevisiae: evidence for protein oxidation and autophagic cell death. Free Radic Biol Med 2005, 38: 226–234.
Rodgers KJ, Hume PM, Dunlop RA, Dean RT: Biosynthesis and turnover of DOPA-containing proteins by human cells. Free Radic Biol Med 2004, 37: 1756–1764.
Grune T, Merker K, Sandig G, Davies KJA: Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun 2003, 305: 709–718.
Bulteau AL, Verbeke P, Petropoulos I, Chaffotte AF, Friguet B: Proteasome inhibition in glyoxal-treated fibroblasts and resistance of glycated glucose-6-phosphate dehydrogenase to 20 S proteasome degradation in vitro . J Biol Chem 2001, 276: 45662–45668.
Stolzing A, Widmer R, Jung T, Voss P, Grune T: Degradation of glycated bovine serum albumin in microglial cells. Free Radic Biol Med 2006, 40: 1017–1027.
Swamy M, Tsai C, Abraham A, Abraham E: Glycation mediated lens crystallin aggregation and cross-linking by various sugars and sugar phosphates in vitro . Exp Eye Res 1993, 56: 177–185.
Ortwerth BJ, Slight SH, Prabhakaram M, Sun Y, Smith JB: Site-specific glycation of lens crystallins by ascorbic acid. Biochimica et Biophysica Acta (BBA)-General Subjects 1992, 1117: 207–215.
Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, Haik GM: Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest Ophthalmol Vis Sci 2003, 44: 5287.
Swamy M, Abraham E: Differential glycation of rat [alpha]-,[beta]-and [gamma]-crystallins. Exp Eye Res 1991, 52: 439–444.
Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K: Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000, 20: 1595–1599.
Lanzer P: Topographic distribution of peripheral arteriopathy in non-diabetics and type 2 diabetics. Z Kardiol 2001, 90: 99–103.
Baynes JW: Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40: 405–412.
Okada E, Oida K, Tada H, Asazuma K, Eguchi K, Tohda G, Kosaka S, Takahashi S, Miyamori I: Hyperhomocysteinemia is a risk factor for coronary arteriosclerosis in Japanese patients with type 2 diabetes. Diabetes Care 1999, 22: 484.
Shuvaev VV, Laffont I, Serot JM, Fujii J, Taniguchi N, Siest G: Increased protein glycation in cerebrospinal fluid of Alzheimer’s disease. Neurobiology of aging 2001, 22: 397–402.
Moreira PI, Smith MA, Zhu X, Nunomura A, Castellani RJ, Perry G: Oxidative stress and neurodegeneration. Ann N Y Acad Sci 2005, 1043: 545–552.
Zhu X, Su B, Wang X, Smith M, Perry G: Causes of oxidative stress in Alzheimer disease. Cellular and molecular life sciences 2007, 64: 2202–2210.
Münch G, Schinzel R, Loske C, Wong A, Durany N, Li J, Vlassara H, Smith M, Perry G, Riederer P: Alzheimer's disease–synergistic effects of glucose deficit, oxidative stress and advanced glycation endproducts. J Neural Transm 1998, 105: 439–461.
Wei Y, Chen L, Chen J, Ge L, He R: Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC cell biology 2009, 10: 10.
Bourdon E, Loreau N, BLACHE D: Glucose and free radicals impair the antioxidant properties of serum albumin. The FASEB journal 1999, 13: 233–244.
Szapacs ME, Riggins JN, Zimmerman LJ, Liebler DC: Covalent adduction of human serum albumin by 4-hydroxy-2-nonenal: kinetic analysis of competing alkylation reactions. Biochemistry 2006, 45: 10521–10528.
Rondeau P, Singh NR, Caillens H, Tallet F, Bourdon E: Oxidative stresses induced by glycoxidized human or bovine serum albumin on human monocytes. Free Radic Biol Med 2008, 45: 799–812.
Iberg N, Flückiger R: Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. Identification of multiple glycosylated sites. J Biol Chem 1986, 261: 13542–13545.
Lapolla A, Fedele D, Reitano R, Aricò NC, Seraglia R, Traldi P, Marotta E, Tonani R: Enzymatic digestion and mass spectrometry in the study of advanced glycation end products/peptides. J Am Soc Mass Spectrom 2004, 15: 496–509.
Wa C, Cerny RL, Clarke WA, Hage DS: Characterization of glycation adducts on human serum albumin by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clin Chim Acta 2007, 385: 48–60.
Frolov A, Hoffmann R: Identification and relative quantification of specific glycation sites in human serum albumin. Anal Bioanal Chem 2010, 397: 2349–2356.
Hinton D, Ames J: Site specificity of glycation and carboxymethylation of bovine serum albumin by fructose. Amino Acids 2006, 30: 425–434.
Ahmed N, Dobler D, Dean M, Thornalley PJ: Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J Biol Chem 2005, 280: 5724.
Zeng J, Davies MJ: Evidence for the formation of adducts and S-(carboxymethyl) cysteine on reaction of α-dicarbonyl compounds with thiol groups on amino acids, peptides, and proteins. Chem Res Toxicol 2005, 18: 1232–1241.
Mostafa AA, Randell EW, Vasdev SC, Gill VD, Han Y, Gadag V, Raouf AA, El Said H: Plasma protein advanced glycation end products, carboxymethyl cysteine, and carboxyethyl cysteine, are elevated and related to nephropathy in patients with diabetes. Mol Cell Biochem 2007, 302: 35–42.
Sattarahmady N, Moosavi-Movahedi AA, Ahmad F, Hakimelahi GH, Habibi-Rezaei M, Saboury AA, Sheibani N: Formation of the molten globule-like state during prolonged glycation of human serum albumin. Biochim Biophys Acta 2007, 1770: 933–942.
Brownlee M: The pathological implications of protein glycation. Clinical and investigative medicine Medecine clinique et experimentale 1995, 18: 275.
Chen S, Cohen MP, Ziyadeh FN: Amadori-glycated albumin in diabetic nephropathy: pathophysiologic connections. Kidney Int 2000, 58: S40-S44.
HASAN NA: Effects of trace elements on albumin and lipoprotein glycation in diabetic retinopathy. Saudi Med J 2009, 30: 1263–1271.
Rubenstein DA, Yin W: Glycated albumin modulates platelet susceptibility to flow induced activation and aggregation. Platelets 2009, 20: 206–215.
Candiloros H, Muller S, Ziegler O, Donner M, Drouin P: Role of albumin glycation on the erythrocyte aggregation: An in vitro study. Diabet Med 1996, 13: 646–650.
Unoki H, Bujo H, Yamagishi S, Takeuchi M, Imaizumi T, Saito Y: Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes research and clinical practice 2007, 76: 236–244.
Brandt R, Nawka M, Kellermann J, Salazar R, Becher D, Krantz S: Nucleophosmin is a component of the fructoselysine-specific receptor in cell membranes of Mono Mac 6 and U937 monocyte-like cells. Biochimica et Biophysica Acta (BBA)-General Subjects 2004, 1670: 132–136.
Roohk HV, Zaidi AR: A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol 2008, 2: 1114.
Hashimoto K, Osugi T, Noguchi S, Morimoto Y, Wasada K, Imai S, Waguri M, Toyoda R, Fujita T, Kasayama S: A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care 2010, 33: 509.
Jeffcoate S: Diabetes control and complications: the role of glycated haemoglobin, 25 years on. Diabetic Med 2004, 21: 657–665.
Kosecki SM, Rodgers PT, Adams MB: Glycemic monitoring in diabetics with sickle cell plus β-thalassemia hemoglobinopathy. The Annals of pharmacotherapy 2005, 39: 1557–1560.
Yoshiuchi K, Matsuhisa M, Katakami N, Nakatani Y, Sakamoto K, Matsuoka T, Umayahara Y, Kosugi K, Kaneto H, Yamasaki Y: Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J 2008, 55: 503–508.
Koga M, Murai J, Saito H, Aoki K, Kanehara H, Bando Y, Morita S, Kasayma S: Glycated albumin levels are higher relative to HbA1c levels in patients with autoimmune acute-onset type 1 diabetes mellitus than in patients with type 2 diabetes mellitus at the time of diagnosis. Diabetes Research Clin Pract 2011, 94: 12–14.
Okumura A, Mitamura Y, Namekata K, Nakamura K, Harada C, Harada T: Glycated albumin induces activation of activator protein-1 in retinal glial cells. Jpn J Ophthalmol 2007, 51: 236–237.
Nagayama H, Inaba M, Okabe R, Emoto M, Ishimura E, Okazaki S, Nishizawa Y: Glycated albumin as an improved indicator of glycemic control in hemodialysis patients with type 2 diabetes based on fasting plasma glucose and oral glucose tolerance test. Biomed Pharmacother 2009, 63: 236–240.
Koga M, Murai J, Saito H, Matsumoto S, Kasayama S: Effects of thyroid hormone on serum glycated albumin levels: Study on non-diabetic subjects. Diabetes Research Clin Pract 2009, 84: 163–167.
Koga M, Matsumoto S, Saito H, Kasayama S: Body mass index negatively influences glycated albumin, but not glycated hemoglobin, in diabetic patients. Endocr J 2006, 53: 387–391.
Nishimura R, Kanda A, Sano H, Matsudaira T, Miyashita Y, Morimoto A, Shirasawa T, Kawaguchi T, Tajima N: Glycated albumin is low in obese, non-diabetic children. Diabetes Research Clin Pract 2006, 71: 334–338.
Cohen MP, Ziyadeh FN: Role of Amadori-modified nonenzymatically glycated serum proteins in the pathogenesis of diabetic nephropathy. J Am Soc Nephrol 1996, 7: 183–190.
Buckow R, Wendorff J, Hemar Y: Conjugation of Bovine Serum Albumin and Glucose under Combined High Pressure and Heat. J Agric Food Chem 2011, 59: 3915–3923.
Munanairi A, O'Banion SK, Gamble R, Breuer E, Harris AW, Sandwick RK: The multiple Maillard reactions of ribose and deoxyribose sugars and sugar phosphates. Carbohydr Res 2007, 342: 2575–2592.
Syrový I: Glycation of albumin: reaction with glucose, fructose, galactose, ribose or glyceraldehyde measured using four methods. Journal of biochemical and biophysical methods 1994, 28: 115–121.
Schmitt A, Schmitt J, Münch G, Gasic-Milencovic J: Characterization of advanced glycation end products for biochemical studies: side chain modifications and fluorescence characteristics. Analytical Biochemistry 2005, 338: 201–215.
GhoshMoulick R, Bhattacharya J, Roy S, Basak S, Dasgupta AK: Compensatory secondary structure alterations in protein glycation. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics 2007, 1774: 233–242.
Barnaby OS, Wa C, Cerny RL, Clarke W, Hage DS: Quantitative analysis of glycation sites on human serum albumin using < sup > 16</sup > O/< sup > 18</sup > O-labeling and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Clin Chim Acta 2010, 411: 1102–1110.
Holm NK, Jespersen SK, Thomassen LV, Wolff TY, Sehgal P, Thomsen LA, Christiansen G, Andersen CB, Knudsen AD, Otzen DE: Aggregation and fibrillation of bovine serum albumin. Biochimica et Biophysica Acta (BBA)-Proteins & Proteomics 2007, 1774: 1128–1138.
Acknowledgements
We thank Dr. Ebrahim-Habibi for her kindly assistance. The financial support of Research Council of Tehran University are acknowledged. Also, authors are grateful of the support by modeling and simulation laboratory of Tehran University of medical sciences in Shariati Hospital.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare to have no competing interests.
Authors’ contributions
AA design of the study, SF helped to draft the manuscript, MH participated in the design of the study and overall manuscript architecture, AM conceived of the study. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Arasteh, A., Farahi, S., Habibi-Rezaei, M. et al. Glycated albumin: an overview of the In Vitro models of an In Vivo potential disease marker. J Diabetes Metab Disord 13, 49 (2014). https://doi.org/10.1186/2251-6581-13-49
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6581-13-49