Abstract
Jatropha curcas L. seeds as a raw material for biodiesel production is a rapidly growing interest over the world because of its high oil content, ecological adaptability, and excellent fuel properties. Though there is an increase in productivity of biodiesel, showing solution for future energy insecurity, there still remains some concern for commercialization due to its susceptibility to degradation during long storage. The aim of this paper is to investigate the effect of temperature and ambient condition on Jatropha biodiesel storage. An experiment was conducted for a period of 12 months, where Jatropha biodiesel stored in three groups at different temperatures (4°C, 25°C, and 35°C) and environmental conditions (exposed in dark, light, and air). At regular intervals, the samples were taken out to analyze acid value, density, kinematic viscosity, and thermogravimetric profile to monitor the quality of biodiesel. Analysis showed that acid value, density, kinematic viscosity, and the onset temperature of volatilization and distillation increases with the increase in storage time of biodiesel samples. However, Jatropha biodiesel stored at 35°C, in contact with ambient air and light showed highest degradation compared to those which were stored at 25°C and 4°C. Among all the parameters studied, high temperature and air exposure are the two most potent parameters which accelerate the degradation process. Along with that, light exposure had mild but significant effect on Jatropha biodiesel degradation over a long storage period.
Similar content being viewed by others
Background
Biodiesel is a monoalkyl ester of long-chain fatty acid derived from vegetable oil or animal fat [1]. It constitutes the most reliable renewable source for substituting diesel. The finite nature of fossil fuel reserves and growing green house gas emission have promoted research on alternative fuels [2, 3]. Biodiesel has advantages over conventional diesel fuels because of its renewable, environmentally benign and biodegradable nature. The main disadvantage is that biodiesels are severely prone to degradation than conventional diesel fuels during long storage [4]. Storage stability is a critical issue regarding the successful commercialization of biodiesel in fuel market. Storage stability is the ability of a fuel to resist changes in its physicochemical characteristics brought by interaction with its environment. In the presence of air or oxygen, biodiesel will be hydrolyzed into alcohol and acid. The presence of alcohol reduces flash point, and the presence of acid increases the total acid value. All these make biodiesel unstable during storage. But in order to introduce biodiesel in the transport industry, it should meet accepted fuel standards and quality assurance [5].
There are various reports in the literature on the storage, oxidation stability, and the effect of antioxidant concentration on biodiesel. The effect of different synthetic and natural antioxidants on oxidation stability of biodiesel produced from rapeseed oil, sunflower oil, used frying oil, beef tallow, and soya bean oil has been reported [6, 7]. Long time storage stability are also investigated on biodiesels synthesized from rapeseed oil, used frying oil, high oleic sunflower oil, high and low erucic Brassica carinata oil [5, 8, 9]. Dunn [10] examined the oxidative stability of soy-bean oil methyl esters by analyzing oil stability index Polavka et al. [11] studied the oxidation stability of rapeseed oil and waste frying oil methyl esters using differential thermal analysis and Rancimat (Metrohm AG, Herisau, Switzerland). Most of the studies on the storage and oxidation stability are carried out on biodiesel derived from edible oil. However, the studies on biodiesel derived from non-edible oil seeds are scanty [12–14]. Das et al. [12] analyzed the effect of light, air exposure, and antioxidants namely propyl gallate, butylatedhydrox-yanisole, and butylatedhydroxytoluene on long-term storage stability of karanja oil methyl ester (KOME). Their study showed that oxidative stability of KOME decreases with the increase in storage time and increases with higher concentration of antioxidant levels.
Among non-edible oil crop, recently, Jatropha is in the top priority in national biodiesel program because of its high oil content and promising fuel properties. Several researchers have performed experiments for efficient Jatropha biodiesel production, physicochemical characterization [15, 16], and engine performance [17]. Sarin et al. [18] monitored the effect of metal contaminants and antioxidant concentrations on the storage stability of Jatropha biodiesel. However, no proper study has been conducted on the effect of different storage parameters like temperature and exposure to light and air on Jatropha methyl ester (JME) storage. The aim of this work is to investigate the influence of different parameters like temperature and exposure to light and air on Jatropha biodiesel degradation. Acid value, density, kinematic viscosity (KV), and thermogravimetric analysis (TGA) results for methyl esters were analyzed for a period of 12 months and were compared with the initial values to monitor the changes in the quality of methyl esters. The study will provide knowledge on the factor influencing degradation of Jatropha biodiesel, which will be immensely helpful in maintaining the quality of Jatropha biodiesel over long-term storage.
Methods
Materials
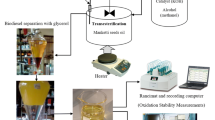
Jatropha seeds were collected from Assam, Northeast India. The seeds were separated from the fruit mechanically and cleaned manually to remove all foreign materials. Then, the seeds were dried under similar temperature (60°C) and humidity conditions to reach constant weight. The oil was extracted from grinded kernels in Soxhlet apparatus using hexane as per the standard American Oil Chemical Society procedure for 8 h. The extract was concentrated in Rotavapor (Sigma-Aldrich, MO, USA). All the other chemicals and reagents like methanol, ethanol, n-hexane, potassium hydroxide, sodium hydroxide, and phenolphthalein indicator were analytical reagent grade and were purchased from M/s Merck (Merck & Co., Inc., NJ, USA).
Preparation of biodiesel from Jatropha oil
Due to low-acid value of the native oil, single-step direct transesterification procedure was followed. JME was synthesized by refluxing the oil at 60°C, employing 1:6 molar ratio of oil to methanol for 1.5 h. KOH (1 wt%) was used as the catalyst. The mixture was stirred using magnetic beads (400 rpm). After completion of the reaction, the mixture was cooled to room temperature and was transferred to a separating funnel, leading to the separation into two phases. The bottom glycerol layer was discarded. The top ester layer was washed several times with warmed Milliporewater (EMD Millipore Corporation, Billerica, MA, USA) to remove all the trace of catalyst, glycerol, and soap. The washed methyl ester was further purified under vacuum on a rotary evaporator.
The storage condition and analysis
The acid value, density, KV, and TGA analysis were performed and recorded as 0 month values after the preparation of Jatropha methyl ester. Then, the methyl ester samples were stored for 12 months in three individual groups at different storage conditions. The groups were made on the basis of different storage temperatures: 4°C, 25°C, and 35°C. Each group consists of three subgroups: in the first subgroup, the samples were kept in sealed and in dark environment. In the second subgroup, the samples were kept in a sealed environment but exposed to light, while in the third one, the samples were kept but exposed to both light and ambient air. The samples from each group (in three replicates) were analyzed periodically at the intervals of 6 months for different fuel quality parameters such as acid value, density, KV, and TGA.
Analysis
Acid value
Acid value denotes free fatty acid content of oil. It is defined as the amount of potassium hydroxide (in mg) required for neutralizing 1 g of oil sample. It was calculated according to AOCS Official method (Te TA-64, 1997), by titrating solution of JME in neutral ethanol with 0.5 N KOH. Phenolphthalein solution was used as an indicator for titration. Acid value was calculated using the following expression:
where N is the normality of accurately standardized sodium hydroxide solution.
Density
The density of a material is defined as mass per unit volume. The densities of the stored JME samples were determined at 25°C, using specific gravity bottle, according to ASTM D4052 method. Density was calculated using the following expression:
Kinematic viscosity
KV is the measure of resistance of a fluid flow against gravity and is determined as the ratio of dynamic viscosity to the density. Dynamic viscosity was measured for stored JME samples with Rheometer (RheoStress RS1, Thermo Electron, Germany) at 40°C. At three different shear rates (50, 100, and 150 s-1), the dynamic viscosities were measured, and the average values were considered. KV was calculated using the following expression:
Thermogravimetric analysis
The thermogravimetric profile of the JME samples was obtained using Thermogravimetric Analyzer (TGA 851e/LF/1100, Mettler, Switzerland). Conventional TGA was performed on 6 ± 0.5 mg samples under high purity nitrogen atmosphere at a flow rate of 40 ml/min. The samples were heated from ambient temperature to 900°C and at constant heating rate of 10°C/min.
Results and discussion
The degradation behavior of JME samples on long storage under different conditions was assessed by monitoring the changes in their physicochemical properties such as acid value, kinematic viscosity, onset temperature of volatilization, and distillation temperature of thermal decomposition with respect to their initial value at 0 month.
Effect of temperature on biodiesel storage
Temperature plays an important role in the degradation of methyl ester. High temperatures accelerate the process of oxidation which results in the increase in density and viscosity of the fuel [19]. The acid value is one of the promising methods to monitor the degradation of methyl ester. Figure 1a,b,c represents the change in the acid value of the JME samples stored under different conditions. The methyl ester degradation shows direct correlation with acid value. Therefore, increase in acid value with storage time indicates gradual degradation of the methyl ester sample. According to ASTM D6751, the standard maximum allowable limit of the acid value for biodiesel is around 0.5 mg KOH/g. At the beginning (i.e., at 0 months), the acid value of the sample was measured to be 0.44 mg KOH/g which is well below the maximum allowable limit. After 6 months of storage, the acid value of the methyl ester sample stored at 35°C was found to be the highest (9.3 mg KOH/g), whereas the sample stored at 4°C showed the lowest value (3.9 mg KOH/g). Further extending the storage period, JME stored at 4°C showed increase in acid value from 0.44 to 4.8 mg KOH/g. For the samples stored at 25°C, increase in the acid value was found to increase from 0.44 to 14.1 mg KOH/g, but the acid value was found to be increased up to 20.1 mg KOH/g for samples stored at 35°C. This clearly indicates that prolonged exposures of methyl ester to high temperature can deteriorate the biodiesel quality, which was also observed in previous studies with canola, palm, soybean, and sunflower methyl ester [4].
Comparison of density of the stored sample with initial value (i.e., 0 month value) over time also gives an idea about the changes in quality of the biodiesel upon extended storage. Figure 2a,b,c shows that with increase in storage time, the density of JME also increases proportionally. The increase in the density of methyl ester is mainly due to the interaction of peroxide molecules which formed due to the degradation of esters [20]. The density values found to be increased for all JME samples kept under extended storage. The initial density values of the samples for 0 months were measured to be around 0.85 kg/m3. After 6 months of storage period, maximum increase in density of the biodiesel stored at 35°C was measured to be 1.9 kg/m3.On the other hand, biodiesel samples stored at 4°C and 25°C, maximum increase in density was observed around 1.15 kg/m3 and 1.6 kg/m3, respectively (Figure 2c). Further extending the storage period, the maximum increase in density of the samples stored at 35°C over 12-months period was measured as 2.3 kg/m3. Whereas, in the case of the other samples stored at 4°C and 25°C,the density increase was from 0.85 to 1.3 kg/m3 and 0.85 to 1.8 kg/m3, respectively. The data clearly revealed the influence of higher temperature on methyl ester degradation. The increase in density of biodiesel is the first indication of methyl ester degradation.
Kinematic viscosity is another suitable method for monitoring the oxidative degradation of methyl ester. Kinematic viscosity tends to increase upon further oxidation due to the formation of secondary oxidative product. Figure 3a,b,c represents the change in kinematic viscosity of Jatropha biodiesel over 12 months of storage. At the beginning, the kinematic viscosity of JME was found to be around 6.10 × 10-6 m2/s (0-month value) which was within the specified range of ASTM D6751 standards. During the storage over a period of 12 months, the kinematic viscosity values of all the methyl ester samples were found to be increased. After 6 months of storage, maximum increase in kinematic viscosity of the JME stored at 35°C was measured to be 9.2 × 10-6 m2/s. On the other hand, biodiesel samples which were stored at 4°C and 25°C, maximum increase in density was found to be around 8 × 10-6 and 8.5 × 10-6 m2/s, respectively (Figure 3c). Further extending the storage period over 12 months, the JME stored at 35°C showed highest viscosity value of 1.48 × 10-5 m2/s compared to 4°C (8.30 × 10-6 m2/s) and 25°C (1.12 × 10-5 m2/s). This increase in viscosity of the samples might be due to the formation of acids and polymeric compound as a result of oxidation, which further can lead to the formation of gums and sediments [5]. The gum and sediment formation is detrimental to engine operation. The increase in kinematic viscosity is a clear indication of methyl ester degradation which was also observed in stability studies in rapeseed and palm oil biodiesel over longer period of storage [21, 22].
Table 1 presents the TGA data of JME stored under different conditions for a period of 12 months. The data shows that with storage time, onset temperature of volatilization and distillation temperature of thermal decomposition also increased. At the beginning (i.e., 0 month), the onset temperature of volatilization of Jatropha biodiesel was around 120°C. In the JME kept at 4°C and 25°C after 6 months of storage, the onset temperature of volatilization has increased to a maximum of up to 139°C and 165°C, respectively. On the other hand, for the JME kept at 35°C after 6 months of storage, the onset temperature of volatilization has increased a maximum of up to 220°C. Further extending the storage period over 12 months, the JME kept at 4°C and 25°C, the onset temperatures of volatilization were found to be increased to maximum of up to 148°C and 173°C, respectively. Whereas, the JME kept at 35°C after 12 months storage, the onset temperature of volatilization has increased a maximum of up to 310°C. The distillation temperature of thermal degradation of JME stored at 35°C is much higher compared to those of the samples which are kept at 25°C and 4°C. The data presented in the Table 1 show that higher temperature speeds up the formation of secondary oxidative product. Exposure to high temperature will help in isomerisation of methylene interrupted poly-unsaturated olefin structure to form more stable high molecular weight polymer [19], which increases thermal stability of biodiesel. Due to the formation of those thermally stable compounds, degraded methyl ester was found to alter the thermal profile compared to initial thermal profile.
Effect of light on biodiesel storage
To study the influence of light on degradation, JME samples were stored in light and as well as in dark conditions. Figure 1a shows the change in acid value of the biodiesel samples stored under dark and sealed conditions, whereas Figure 1b represents acid value change for samples exposed to light and sealed condition. After 6 months of storage, the samples stored at 4°C under dark and sealed conditions were analyzed for acid value. The acid value of the sample kept in dark condition was found to be increased from 0.44 to 0.46 mg KOH/g. Further extending the storage period over 12 months, the increase in acid value of JME was up to 0.58 mg KOH/g. On the other hand, for the sample stored over a period of 6 months at 4°C in light and sealed condition, increase in the acid value was measured as 0.44 to 0.50 mg KOH/g. After, 12 months of storage, the acid value further increased up to 0.80 mg KOH/g. In the samples stored at 25°C over a period of 12 months in sealed and dark condition, significant increase in the acid value (i.e., 0.44 to 1.5 mg KOH/g) was observed. Whereas, in the same sample stored in sealed condition but exposed to light, the acid value after 12 months of extended storage increases from of 0.44 to 1.8 mg KOH/g. The acid value of methyl ester after 12 months of storage which was kept at 35°C under dark and sealed condition increases from 0.44 to 5.0 mg KOH/g; whereas, in light and sealed condition, the acid value increased from 0.44 to 6.0 mg KOH/g. From this, it is clear that prolonged exposure of light on JME samples fastens the degradation rate compared to the dark condition. This finding was found to be in agreement with the previously published report on degradation of rapeseed biodiesel, where the sample kept in exposure to light showed higher degradation than in dark condition [21].
Increase in the formation of degraded product increases the density and kinematic viscosity of methyl ester. Figure 2a represents the changes in density of stored sample in sealed and dark condition, and Figure 2b is for the sample in sealed but exposed to light. The density of the biodiesel samples kept in dark condition was increased to a maximum of up to 1.0 and 1.5 kg/m3 in 6 and 12 months of storage, respectively. On the other hand, the density of the biodiesel samples in sealed and light condition increased to a maximum of up to 1.4 and 1.9 kg/m3 in 6 and 12 months of storage, respectively. Kinematic viscosity also followed the same pattern. The kinematic viscosity of the biodiesel samples stored in dark and sealed condition has increased to a maximum of up to 7.6 × 10-6 and 8.7 × 10-6 m2/s for 6 and 12 months of storage, respectively; whereas, the kinematic viscosity of the biodiesel sample stored in light increased to a maximum of up to 8.1 × 10-6 and 9.1 × 10-6 m2/s in 6 and 12 months, respectively. The above result gives clear evidence in support of evolution of higher density and kinematic viscosity of stored methyl ester in light in long periods than in dark condition.
Like the other parameters, the effect of temperature on thermal decomposition has also been studied in detail (Table 1). To understand the sample behavior, thermogravimetric analysis was performed for the samples stored under different conditions. The onset temperature of volatilization of 0 month sample was around 120°C. Whereas, for the sample stored over 12 months in light and sealed conditions, the onset temperature of volatilization increased to a maximum of up to 162°C. But, it remains at 138°C for the sample kept in dark and sealed conditions. The distillation temperature required for thermal decomposition of biodiesel kept in light was higher than the sample kept in dark (Table 1). Therefore, from the TGA results, it can be concluded that although the sample stored in light and dark conditions showed closed pattern of degradation, the degradation rate was significantly higher for the samples stored in light than in the dark condition.
Effect of air exposure on biodiesel storage
Air exposure enhances the degradation of methyl esters. During the oxidation process of methyl esters, it usually forms a radical next to double bond which quickly binds with oxygen in air [23]. This forms peroxide, which immediately forms new radical, and thus, the process of radical auto-oxidation cycle starts which exponentially increases the degradation of methyl esters. Figure 1c shows the change in acid value of the JME sample stored in exposure to air. At the beginning (0 month), the acid value of JME was around 0.44 mg KOH/g. After 6 months of storage, the acid value increased from 0.44 to 3.9 mg KOH/g for the samples which are kept at 4°C and exposed to air; whereas, for the sample stored at the same temperature but under sealed condition, increase was not significant, i.e., 0.5 mg KOH/g (Figure 1b,c). Further extending the storage period over 12 months, the increase in acid value was up to 4.8 mg KOH/g for the sample which was kept in exposure to air; whereas, maximum increase in the acid value was found up to 0.8 mg KOH/g for the sealed sample (Figure 1b,c). The samples which were kept at 25°C in exposure to air, the acid value after 6 months of storage was also found to increase from 0.44 to 6.37 mg KOH/g (Figure 1c). On the other hand, maximum increase in the acid value was up to 1.4 mg KOH/g for air-tight sample (Figure 1b). Over 12 months of storage, the increase in acid value was up to 14.1 mg KOH/g for air-exposed JME sample and 1.8 mg KOH/g for air-tight sample stored at 25°C (Figure 1b,c). On the other hand, the samples that are stored at 35°C and exposed to air, the acid value after 6 months of storage was found to increase to 9.3 mg KOH/g; whereas, in sealed condition, the maximum increase was up to 4 mg KOH/g (Figure 1b,c). In this case, also after 12 months of storage, the increase in acid value was up to 20.1 mg KOH/g for air-exposed sample. For the sealed sample, the maximum increase in acid value was up to 6 mg KOH/g (Figure 1b,c). The results obtained showed that air exposure makes biodiesel more susceptible to degradation. When the stored JME samples were compared, the rate of degradation was found to be significantly less for the air-tight samples than that for the air-exposed samples.
Figure 2c represents the change in density of stored JME but exposed to air for a period of 12 months. Density values were also found to follow the same pattern like the acid value of the stored JME sample. For all the samples, the density value was found to be increased with increased in storage time. After 6 months of storage at 4°C, the density of the air-exposed sample was found to be increased from 0.85 (0 months) to 1.15 kg/m3 (Figure 2c). On the other hand, no significant change in the density value was observed (i.e., 0.85 (0 months) to 0.89 kg/m3) for the samples stored in air-tight condition (Figure 2b). After, 12 months of storage, the density increased up to 1.3 kg/m3 for samples that were kept but exposed to air and reached a maximum of up to 0.99 kg/m3 for sealed samples (Figure 2b,c). The density values were found to be increased for the samples that were kept in exposure to ambient air than those for the samples in sealed condition for all storage temperature considered in this study (4°C, 25°C, or 35°C). Result obtained showed that air exposure increases the methyl ester degradation over long storage period.
Figure 3c represents the change in kinematic viscosity of Jatropha biodiesel over 12 months of storage period. Kinematic viscosities of all samples were found to be increased with increase in storage period. When the kinematic viscosity of JME samples stored in exposure to air and air-tight condition compared, significant difference was observed. After 6 months of storage, the JME samples that were kept at 4°C in exposure to air, the kinematic viscosity was to increase from 6.1 × 10-6 (initial value) to 8 × 10-6 m2/s (Figure 3c); whereas, in sealed condition, the value increased to a maximum of up to 7.2 × 10-6 m2/s (Figure 3b). After 12 months of storage, the increase in kinematic viscosity was up to 8.3 × 10-6 m2/s for air-exposed sample, and for air-tight sample, a maximum increase was up to 7.5 × 10-6 m2/s (Figure 3b,c). The samples that were kept at 25°C and 35°C in exposure to air, the kinematic viscosities after 6 months of storage were also found to be increased up to 8.5 × 10-6and 9.2 × 10-6 m2/s, respectively (Figure 3c);whereas, in sealed condition, a maximum increase in kinematic viscosity was up to 7.8 × 10-6and 8.1 × 10-6 m2/s for the samples kept at 25°C and 35°C, respectively (Figure 3b). Similarly, after 12 months of storage, increase in the kinematic viscosity was found to be 1.12 × 10-5and 1.48 × 10-5 m2/s for samples kept in exposure to air at 25°C and 35°C, respectively (Figure 3c). On the other hand, for the same sample kept in sealed condition, the increase in kinematic viscosity was found up to 8.2 × 10-6 and 9.1 × 10-6 m2/s at 25°C and 35°C, respectively (Figure 3b). The result clearly revealed the detrimental effect of air exposure on the quality of methyl esters over long storage period, which was also observed in long-storage stability of vegetable oil and used frying oil-based biodiesel [5].
The onset temperature of volatilization and distillation temperature of thermal decomposition for all JME samples were also increased with increase in storage time. However, temperature required for thermal decomposition of JME samples exposed to ambient air was higher than that of the sample stored in sealed condition over a period of 12 months (Table 1). The initial onset temperature of volatilization for JME was around 120°C. The onset temperature of volatilization increased up to 148°C for JME samples that were exposed to air (stored at 4°C). It only raised maximum up to 138°C for air-tight sample stored at the same temperature (i.e., 4°C). JME samples stored at 25°C and 35°C also showed similar pattern (Table 1). For the storage period of 12 months, the onset temperature of volatilization increased up to 173°C for air-exposed samples, and a maximum increase was up to 162°C for air-tight samples stored at 25°C. On the other hand, for the sample stored at 35°C over 12 months, the onset temperature of volatilization increased up to 310°C for air-exposed JME samples. For air-tight samples stored at 35°C, maximum increase in onset temperature of volatilization was noted as 280°C. The distillation temperature required for thermal decomposition of biodiesel was also found to be higher for the sample exposed to air than that for the sample kept in sealed condition (Table 1). This increase in thermal stability of degraded biodiesel was mainly due to the decomposition of hydroperoxide which forms high molecular weight secondary oxidative product like aldehydes. Air exposure catalyzes the formation of this thermo stable secondary oxidative product. From the above discussion, it is clear that air exposure was one of the driving factors for worsening the quality of Jatropha biodiesel over long-term storage.
Conclusions
For successful commercialization of Jatropha biodiesel, maintaining the quality of biodiesel during storage period is of considerable significance. The study investigated experimentally the effect of storage parameter on Jatropha biodiesel stability. Results showed that JME quality deteriorates over long storage period. Increase in the value of qualitative parameters such as acidity, density, kinematic viscosity, onset temperature of volatilization, and distillation proves that the process of degradation increases with the increase in storage period. JME deterioration was more pronounced at 35°C compared to 25°C and 4°C. The degradation levels of Jatropha biodiesel stored at 25°C and 4°C were of similar pattern and less than the biodiesel stored at 35°C. Increase in the acid value of biodiesel stored at 4°C (from 0.44 to 4.8 mg KOH/g) and 25°C (from 0.44 to 14.1 mg KOH/g) was found to be moderate, while increase in the acid value was abrupt for the sample stored at 35°C (from 0.44 to 20.1 mg KOH/g). This abrupt increase in the acid value was observed for the sample stored in condition exposed to ambient air. In the analysis of the other two parameters, the density and kinematic viscosity also support this result. The maximum increase in density value was found to be from 0.85 to 1.3 kg/m3 in case of 4°C storage temperature, from 0.85 to 1.8 kg/m3 for 25°C, and for 35°C, it increased up to 2.3 kg/m3 for the sample kept but exposed to air. The kinematic viscosity of biodiesel kept but exposed to air increased from 6.1 × 10-6 m2/s to maximum of 1.48 × 10-5 m2/s for sample stored at 35°C, and 1.12 × 10-5 m2/s for sample stored at 25°C; whereas, in case of the sample stored at 4°C, it only increases slightly up to 8.3 × 10-6 m2/s. Although light exposure did not show profound effect on biodiesel degradation, slight significant difference was observed in degradation level between the Jatropha biodiesel kept in light and in dark. The TGA data also showed higher onset temperature of volatilization and distillation temperatures for sample kept at 35°C compared to sample kept at 25°C and 4°C;the degradation was significantly higher in air-exposed condition than air-tight condition. Comparing all the data obtained from the analysis, it can be concluded that among all storage parameters, high temperature and air exposure were the two strong factors in fastening the Jatropha biodiesel degradation. Proper care should be taken for these parameters during storage; otherwise, biodiesel may become unusable very soon.
Authors’ information
PM is a Ph.D. scholar at the Center for Energy, Indian Institute of Technology Guwahati. Her focus on research lies in biofuel production and genetic improvement studies in biofuel plants. VBB holds masters degree in Chemical Engineering. He is currently pursuing his Ph.D. at the Department of Chemical Engineering, Indian Institute of Technology Guwahati in the area of biofuels and biolubricants processing. LS is working as professor at IIT Guwahati. His research interests include genetic manipulation of crops for stress tolerance. VVG is working as an assistant professor at IIT Guwahati. His research interests are in the area of biofuel development and bioconversion of agricultural crops and wastes to useful products.
References
Raj MT, Kandasamy MKK: Tamanu oil - an alternative fuel for variable compression ratio engine. Int. J. Energy. Env. Eng. 2012, 3: 8. 10.1186/2251-6832-3-8
Betiku E, Adepoju TF: Methanolysis optimization of sesame ( Sesamumindicum ) oil to biodiesel and fuel quality characterization. Int. J. Energy Env. Eng. 2013, 4: 9. 10.1186/2251-6832-4-9
Eddine BT, Salah MM: Solid waste as renewable source of energy: current and future possibility in Algeria. Int. J. Energy Env. Eng. 2012, 3: 17.
Moser BR: Influence of extended storage on fuel properties of methyl esters prepared from Canola, palm, soybean and sunflower oils. Renew. Energy 2011, 36: 1221–1226. 10.1016/j.renene.2010.10.009
Bouaid A, Martinez M, Aracil J: Long storage stability of biodiesel from vegetable and used frying oils. Fuel 2007, 86: 2596–2602. 10.1016/j.fuel.2007.02.014
Mittelbach M, Schober S: The influence of antioxidants on the oxidation stability of biodiesel. J. Am. Oil Chem. Soc. 2003, 80(8):817–823. 10.1007/s11746-003-0778-x
Dunn RO: Effect of antioxidants on oxidative stability of methyl soyate. Fuel Process. Technol. 2005, 86(10):1071–1085. 10.1016/j.fuproc.2004.11.003
Mittelbach M, Gangl S: Long storage stability of biodiesel made from rapeseed and used frying oil. J. Am. Oil. Chem. Soc. 2001, 78(6):573–577. 10.1007/s11746-001-0306-z
Bondioli P, Gasparoli A, Bella LD: Biodiesel stability under commercial storage conditions over one year. Eur. J. Lipid Sci. Technol 2003, 105(12):735–741. 10.1002/ejlt.200300783
Dunn RO: Oxidative stability of soybean oil fatty acid methyl esters by oil stability index (OSI). J. Am. Oil Chem. Soc. 2005, 82(5):381–387. 10.1007/s11746-005-1081-6
Polavka J, Paligova J, Cvengros J, Simon P: Oxidation stability of methyl esters studied by differential thermal analysis and Rancimat. J. Am. Oil Chem. Soc. 2005, 82(7):519–524. 10.1007/s11746-005-1103-4
Das LM, Bora DK, Pradhan S, Naik MK, Naik SN: Long-term storage stability of biodiesel produced from Karanja oil. Fuel 2009, 88: 2315–2318. 10.1016/j.fuel.2009.05.005
Diwani GE, Rafie SE, Hawash S: Protection of biodiesel and oil from degradation by natural antioxidants of Egyptian Jatropha. Int. J. Environ. Sci. Tech 2009, 6(3):369–378.
Jain S, Sharma MP: Long term storage stability of Jatropha curcas biodiesel. Energy 2011, 36: 5409–5415. 10.1016/j.energy.2011.06.055
Lu H, Liu Y, Zhou HZ, Yang Y, Chen M, Liang B: Production of biodiesel from Jatropha curcas L. oil. Comp. Chem. Eng. 2009, 33: 1091–1096. 10.1016/j.compchemeng.2008.09.012
Berchmans HJ, Hirata S: Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresour. Technol. 2008, 99: 1716–1721. 10.1016/j.biortech.2007.03.051
Agarwal D, Agarwal AK: Performance and emissions characteristics of Jatropha oil (preheated and blends) in a direct injection compression ignition engine. Appl. Therm. Eng. 2007, 27: 2314–2323. 10.1016/j.applthermaleng.2007.01.009
Sarin A, Arora R, Singh NP, Sharma M, Malhotra RK: Influence of metal contaminants on oxidation stability of Jatropha biodiesel. Energy 2009, 34: 1271–1275. 10.1016/j.energy.2009.05.018
Jain S, Sharma MP: Stability of biodiesel and its blends: a review. Renew. Sustain. Energ. Rev. 2010, 14: 667–678. 10.1016/j.rser.2009.10.011
Pattamaprom C, Pakdee W, Ngamjaroen S: Storage degradation of palm-derived biodiesels: its effects on chemical properties and engine performance. Renew. Energ. 2012, 37: 412–418. 10.1016/j.renene.2011.05.032
Leung DYC, Koo BCP, Guo Y: Degradation of biodiesel under different storage condition. Bioresour. Technol. 2006, 97: 250–256. 10.1016/j.biortech.2005.02.006
Lin C-Y, Chiu C-C: Effects of oxidation during long term storage on the fuel properties of palm-oil based biodiesel. Energy Fuel 2009, 23: 3285–3289. 10.1021/ef900105t
Bora DK, Das LM, Babu MKG: Storage stability of mahua oil methyl ester. J. Sci. Indus. Res. 2009, 68: 149–158.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
PM and VBB carried out the study plan and were involved in the laboratory work. VVG and LS initiated, guided, and supervised the research work. VVG reviewed the revised manuscript and helped in correcting the manuscript. All the authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mazumdar, P., Borugadda, V.B., Goud, V.V. et al. Effect of storage parameters on stability of Jatropha-derived biodiesel. Int J Energy Environ Eng 4, 13 (2013). https://doi.org/10.1186/2251-6832-4-13
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-6832-4-13