A growing body of evidence supports the efficacy and likely cost-effectiveness of adjunctive psychosocial interventions for bipolar disorder through their capacity to reduce rates of relapse. Reference Scott, Colom and Vieta1–Reference Zaretsky, Lancee, Miller, Harris and Parikh5 Despite these advances, few studies have rigorously assessed group-based therapy as a mode of treatment delivery. Only three published randomised controlled trials have evaluated group therapy alone for people with bipolar disorder, one being the pilot of the study described here. Reference Colom, Vieta, Martinez-Aran, Reinares, Goikolea and Benabarre6–Reference Scott10 We report the findings of a randomised controlled trial undertaken in a naturalistic setting which examined the efficacy of a manualised group-based intervention as an adjunctive treatment for people with bipolar disorder. We predicted that compared with treatment as usual, participation in the group-based intervention would decrease the number and duration of bipolar relapses of any type. A secondary hypothesis was that the intervention would have a positive impact on psychiatric symptoms.
Method
Trial design
In a randomised controlled trial a manualised group-based intervention plus treatment as usual was compared with treatment as usual as the control condition. The trial was conducted in metropolitan and regional sites in Victoria, Australia, between 2004 and 2007. Human research ethics approval was obtained from each site. Following referral, all participants were contacted by a research clinician who explained the project using a plain language statement and answered any questions prior to obtaining informed consent. All participants were assessed at baseline and then randomised to either treatment or control. All participants were followed up for 9 months after completion of the 3-month intervention period.
Participants
A total of 84 persons meeting DSM–IV–TR criteria for bipolar disorder were recruited to participate in this study. 11 Inclusion criteria for the study were as follows: a diagnosis of bipolar affective disorder type 1, 2 or not otherwise specified; age 18–65 years; ability to converse in English without an interpreter; under the care of a medical practitioner (general practitioner or psychiatrist) and not in an acute episode as defined by DSM–IV–TR criteria for mania or depression. Exclusion criteria were limited to the presence of diagnosed developmental disability or cognitive impairment sufficient to preclude involvement in the intervention.
To facilitate maximum inclusion of participants in a naturalistic setting, individuals were recruited from a variety of settings, including referral from their service provider (public sector, private sector or primary care). To assist in recruitment, written and verbal information about the study and how to participate was disseminated through general practitioners, mental health services and a media release.
Randomisation
Randomisation was achieved using balanced allocation tables for each site generated by the statistician using www.randomization.com and concealed in a password-protected area of the computer system. When a group of 10–14 participants at a given site had signed consent, their names were given to the statistician for block simultaneous randomisation in order of date of consent. The statistician had no knowledge of the referral details of participants when allocating them to the treatment or control condition.
Assessment procedure
Face-to-face interviews were conducted at baseline, after the intervention period (3 months post-baseline) and at 12 months post-baseline. The research clinicians conducting face-to-face interviews were unaware of group allocation. Each interview took approximately 90 min. To acknowledge the time given up by participants for follow-up assessment, they were reimbursed with a nominal AU$20 gift voucher for each post-intervention interview; those in the intervention group were not paid for participation in the therapy itself.
Telephone interviews, limited to 20 min duration, were conducted monthly by research clinicians during the 9-month follow-up period to collect data regarding relapses. Budgetary limitations meant that it was not feasible for the researchers conducting the large number of follow-up telephone interviews to remain unaware of allocation. However, fidelity of data collection was ensured using detailed telephone records that were randomly selected for auditing by a senior research clinician masked to treatment or control condition.
Baseline data
The Mini-International Neuropsychiatric Interview 5.0 was used to confirm psychiatric diagnosis and identify comorbidities. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller12 Demographic information regarding the participant's living circumstances, past psychiatric history and mental health service use data were also collected.
Outcome measures
Primary outcomes
The primary outcomes were three parameters of relapse (see Statistical analysis) of any mood episode occurring in the 9-month follow-up period. Relapse was defined as meeting DSM–IV–TR criteria for any mood episode (mania, hypomania, depression, mixed, other). A structured telephone interview based on DSM–IV–TR criteria was used to determine whether any relapses had occurred in the previous month, and if so, the type and duration. Telephone interviews have been shown to be a feasible and reliable method for collection of data regarding bipolar diagnoses. Reference Revicki, Tohen, Laszlo, Thompson, Pike and Davis-Vogel13
Secondary outcomes
Secondary outcomes were assessed using structured clinical interviews. Psychiatric symptoms were measured using clinician-rated scales including the Montgomery–Åsberg Depression Rating Scale (MADRS) Reference Montgomery and Åsberg14 and the Young Mania Rating Scale (YMRS). Reference Young, Biggs, Ziegler and Meyer15
Intervention
All participants were under the care of a medical practitioner (general practitioner or psychiatrist) and usual medications were continued throughout the study. At baseline, participants in both the intervention and the control groups were equally distributed under psychiatric or general practitioner care. Both groups had access to multidisciplinary specialist mental health services if they became unwell. This is consistent with usual protocols within the mental health system in Victoria, which has a uniform structure throughout the state, and reflects typical patterns of service use by patients with bipolar disorder. Reference Morgan, Mitchell and Jablensky16
Control: treatment as usual plus telephone calls
The control condition consisted of treatment as usual and weekly telephone calls. These calls were made during the initial 12-week intervention period to maintain engagement in the trial and to control for this aspect of facilitator contact time in the treatment condition.
Treatment: group programme plus treatment as usual
The treatment condition consisted of treatment as usual plus a structured group programme comprising an initial block of 12 weekly sessions with three additional monthly booster sessions to support participants in applying knowledge and skills to everyday life situations (Appendix). Weekly telephone calls were incorporated into the 12-week programme during the initial intervention period to remind participants of the next group session and to offer support for any homework tasks assigned.
The programme was developed using the Collaborative Therapy Framework and is based on a stress vulnerability model. Reference Castle and Gilbert17 This framework involves comprehensive assessment and structured programmes designed to enable participants to optimise their health and prevent relapse by developing and maintaining coping strategies to address vulnerabilities and manage stress. The programme developed for this study integrates effective coping strategies from existing psychosocial approaches, including monitoring mood and activities (M), assessing prodromes (A), preventing relapse (P) and setting Specific, Measurable, Achievable, Realistic, Time-framed (SMART) goals (S), and is known by the acronym MAPS. A number of resources, including a participant workbook, information book and a collaborative therapy journal (a client-held record) were used throughout the programme to reinforce and enhance skill development, promote self-efficacy and develop effective relationships between the participants and their service providers. For a detailed outline of group programme content and resources, see Castle et al. Reference Castle, Berk, Berk, Lauder, Chamberlain and Gilbert7
Each group had a maximum of seven participants and each session was of 90 min duration. All groups were facilitated by one senior research clinician from the project team and a clinician recruited from a clinical site involved in the study. Senior research clinicians had professional qualifications in psychology, occupational therapy, psychiatric nursing, social work or psychiatry; a minimum of 3 years' experience working in clinical mental health settings; and previous group facilitation experience. All facilitators attended a 1-day training workshop in the use of the manualised treatment and received weekly supervision to ensure fidelity to the treatment manual.
Statistical analysis
The number of participants required to detect a clinically meaningful hazard ratio for relapse of 0.4 with statistical power greater than 0.8 was estimated to be 50 persons for the treatment condition and 50 persons for the control condition. We aimed to recruit 60 participants for each condition, thereby allowing for a 20% attrition rate. Reference Halperin, Nathan, Drummond and Castle18 The analysis was based on the intention-to-treat principle in that information from all participants was included in the analysis, either directly or through a missing data handling procedure, according to their allocated condition. Stata version 10 for Windows was used for all analyses.
The primary outcome measure was based on pooled data for any type of relapse (depressive, manic, mixed, hypomanic or other). The treatment and control groups were compared on three parameters of relapse:
-
(a) survivor function for first relapse of any type;
-
(b) occurrence of no relapse v. at least one relapse per participant as a binary measure using Fisher's exact test;
-
(c) fraction of time spent unwell during the 9-month follow-up period using the Mann–Whitney test.
To estimate the survivor function, Kaplan–Meier survival curves were plotted and a log rank test used to assess the equality of these curves. Cox regression was then used to estimate a hazard ratio for treatment v. control with the proportional hazard assumption checked using Schoenfeld residuals. To explore further specific subtypes of relapse, subsequent analyses using the same three parameters of relapse were performed where possible, first for depression and then for mania and mixed episodes.
The repeated-measures data for secondary outcomes (psychiatric symptoms) were analysed using generalised estimating equations. Reference Hardin and Hilbe19 The measure of treatment effect was the coefficient of the interaction between the treatment allocation and whether the data were acquired at baseline or post-baseline. Data from participants who withdrew after baseline were accounted for by making a ‘missing at random’ assumption using the three covariates that predicted whether outcomes were missing or present. These three covariates were included in the relevant regressions to adjust for the missing outcome data. Reference Carpenter and Kenward20 Missing data in the covariates were imputed using multiple imputation, Reference Carlin, Galati and Royston21 with ten imputations.
Results
In total, 117 people were screened for potential involvement in the study and, of these, 84 consented to participate. The treatment and control groups were similar at baseline in terms of demographic and clinical parameters (Table 1). The presence of lifetime or current anxiety or eating disorders was the only significant difference, with the greater comorbidity in the treatment group. There was no significant difference in the referral source or pharmacotherapy received between groups, suggesting that at baseline, treatment as usual was comparable for both treatment and control participants. The numbers of admissions 12 months prior to baseline were similar in both treatment and control groups: mean 1.02 (s.d. = 1.30) v. 0.76 (s.d. = 1.34); Mann–Whitney z = –1.24, P = 0.2. The number of weeks spent in hospital in the 12 months prior to baseline was also similar in the two groups: treatment group mean 2.33 weeks (s.d. = 5.90), control group mean 2.14 weeks (s.d. = 4.89); Mann–Whitney z = –0.47, P = 0.6.
Table 1 Characteristics of the participants at baseline
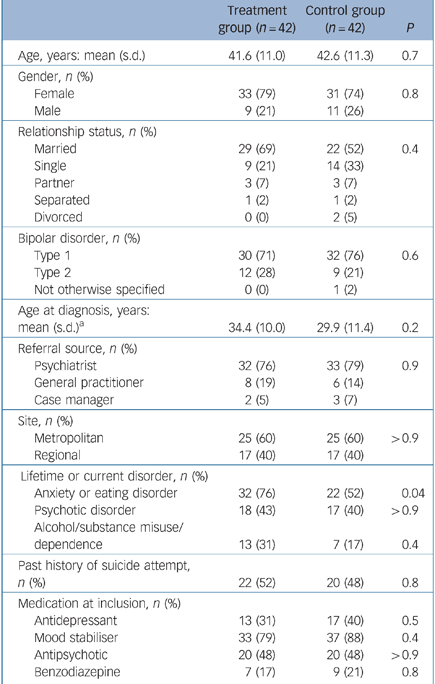
| Treatment group (n = 42) | Control group (n = 42) | P | |
|---|---|---|---|
| Age, years: mean (s.d.) | 41.6 (11.0) | 42.6 (11.3) | 0.7 |
| Gender, n (%) | |||
| Female | 33 (79) | 31 (74) | 0.8 |
| Male | 9 (21) | 11 (26) | |
| Relationship status, n (%) | |||
| Married | 29 (69) | 22 (52) | 0.4 |
| Single | 9 (21) | 14 (33) | |
| Partner | 3 (7) | 3 (7) | |
| Separated | 1 (2) | 1 (2) | |
| Divorced | 0 (0) | 2 (5) | |
| Bipolar disorder, n (%) | |||
| Type 1 | 30 (71) | 32 (76) | 0.6 |
| Type 2 | 12 (28) | 9 (21) | |
| Not otherwise specified | 0 (0) | 1 (2) | |
| Age at diagnosis, years: mean (s.d.)a | 34.4 (10.0) | 29.9 (11.4) | 0.2 |
| Referral source, n (%) | |||
| Psychiatrist | 32 (76) | 33 (79) | 0.9 |
| General practitioner | 8 (19) | 6 (14) | |
| Case manager | 2 (5) | 3 (7) | |
| Site, n (%) | |||
| Metropolitan | 25 (60) | 25 (60) | > 0.9 |
| Regional | 17 (40) | 17 (40) | |
| Lifetime or current disorder, n (%) | |||
| Anxiety or eating disorder | 32 (76) | 22 (52) | 0.04 |
| Psychotic disorder | 18 (43) | 17 (40) | > 0.9 |
| Alcohol/substance misuse/dependence | 13 (31) | 7 (17) | 0.4 |
| Past history of suicide attempt, n (%) | 22 (52) | 20 (48) | 0.8 |
| Medication at inclusion, n (%) | |||
| Antidepressant | 13 (31) | 17 (40) | 0.5 |
| Mood stabiliser | 33 (79) | 37 (88) | 0.4 |
| Antipsychotic | 20 (48) | 20 (48) | > 0.9 |
| Benzodiazepine | 7 (17) | 9 (21) | 0.8 |
Flows through the study are shown in Fig. 1. Overall retention rates were high in both treatment and control conditions. After randomisation, reasons for not commencing the allocated condition included being unavailable owing to work or study commitments (n = 2) and an episode of illness (n = 1) in the treatment group, and an episode of illness (n = 1) in the control group. During the intervention phase, reasons for withdrawing included work or study commitments (n = 3), episode of illness (n = 2), travel overseas (n = 1) and lost contact (n = 1) in the treatment group, and lost contact (n = 1) in the control group. No participant withdrew from the study during the follow-up phase.
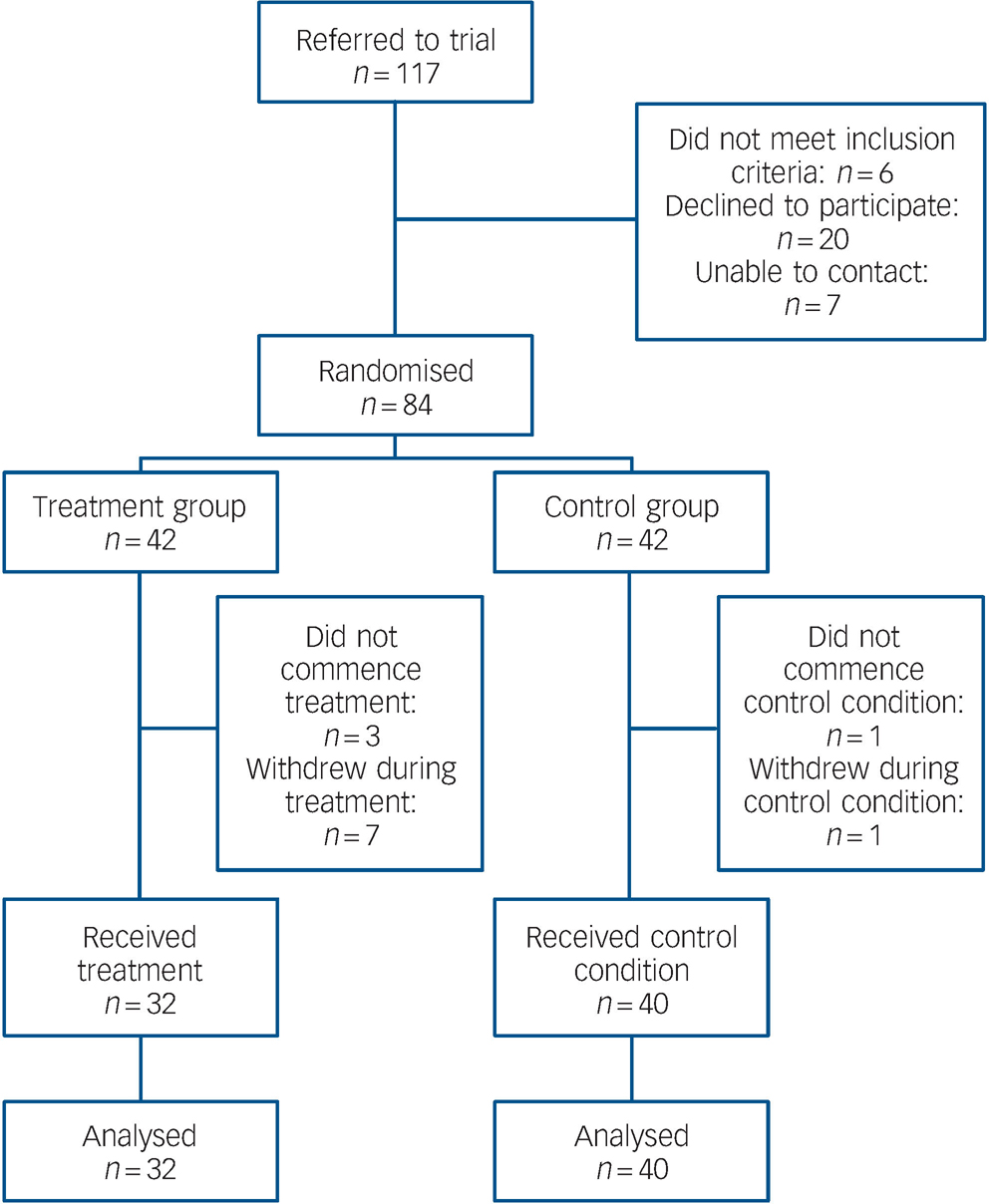
Fig. 1 Flow of participants through trial.
Relapse
As we were interested in the impact of the group-based programme on relapse, only relapses that occurred in the 9 months after the initial intervention period or equivalent for the control condition are considered here. The distribution of the relapses occurring in treatment and control groups, including multiple relapses for some participants, is as follows: depression, 4 in treatment and 15 in control; mania, 0 in treatment and 6 in control; hypomania, 9 in treatment and 5 in control; mixed, 1 in treatment and 1 in control; other (e.g. overdose, self-harm), 2 in treatment and 2 in control; total, 16 in treatment and 29 in control.
Survival curves for all first relapses of any type are presented in Fig. 2. The difference between treatment and control curves is statistically significant (log rank test χ2(1) = 4.31, P = 0.04) An adjusted Cox regression indicated significantly lower rates of relapse (pooled over any type) in the treatment group (hazard ratio (HR) = 0.43, 95% CI 0.20–0.95; t 343 = –2.09, P = 0.04). There was a significantly greater number of participants in the control group who had at least one relapse of any type (no relapse: 23 participants in treatment and 18 in control; one or more relapses: 9 in treatment and 22 in control, Fisher's exact test, two-sided, P = 0.03). The fraction of time spent unwell in any type of relapse for treatment participants (mean = 0.041) was compared with the fraction of time spent unwell for control participants (mean = 0.087). The treatment participants spent significantly less time unwell on average (Mann–Whitney z = 2.29, P = 0.02). In summary, all three analyses of relapse (of any type) were in the direction of the primary hypothesis and all found significant between-group differences.
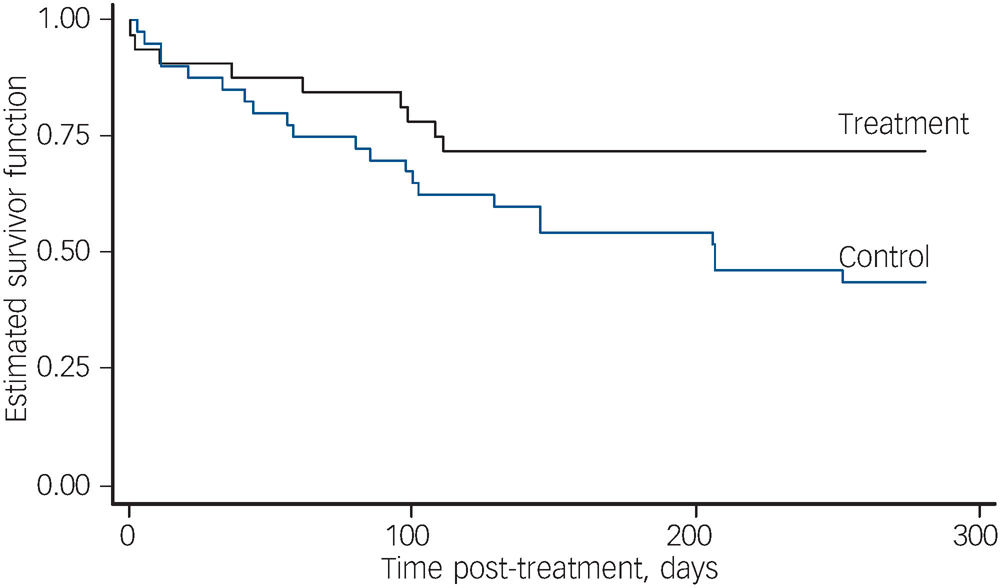
Fig. 2 Survival estimates for first relapse of any type.
Subtypes of relapse
Subsequent analyses were performed on depression and on mania and mixed pooled subtypes of relapse.
Depression. For the survival curves for the first relapse of depression, the log rank test showed a significant difference between the treatment and control conditions (χ2(1) = 5.53, P = 0.02). An adjusted Cox regression indicated that there were significantly fewer depressive relapses in the treatment group (HR = 0.18, 95% CI 0.04–0.85; t 545 = –2.16, P = 0.03). There was a significantly greater number of participants with one or more depressive relapses in the control group (two-sided Fisher's exact test P = 0.03). The fraction of time spent unwell in a depressive relapse was greater on average for the control group (mean = 0.067) than for the treatment group (mean = 0.028; Mann–Whitney z = 2.27, P = 0.02).
Mania and mixed pooled subtypes. Overall, there were more cases of mania and mixed relapse in the control group than in the treatment group. None of the first relapses in the treatment group were of mania or mixed type. The log rank test for the survivor functions for first relapse of mania or mixed episode gave a significant result (χ2(1) = 6.65, P = 0.01). Although this indicates a significant difference between treatment and control, with no first relapse of mania or mixed type in the treatment group the hazard ratio cannot be estimated.
Secondary outcomes
At baseline there was no significant difference in depressive symptoms between the treatment and control groups. The MADRS mean total score was 15.0 (s.d. = 10.2) for the treatment group v. 11.4 (s.d. = 9.8) for the control group (P = 0.12). At the 3-month follow-up assessment the mean scores were 10.6 (s.d. = 7.6) v. 9.4 (s.d. = 8.5) and at 12 months they were 11.2 (s.d. = 9.8) v. 7.5 (s.d. = 8.5). The adjusted treatment effect coefficient was –0.61 (95% CI –4.42 to 3.20, t 32 = –0.32, P = 0.8), indicating that the post-treatment scores did not show a significant difference between treatment and control. Similarly, there was no significant difference in manic symptoms at baseline: mean YMRS scores were 6.1 (s.d. = 5.3) v. 5.9 (s.d. = 6.1); P = 0.9. The 3-month scores were 4.7 (s.d. = 4.5) v. 3.6 (s.d. = 4.0) and the 12-month scores were 4.5 (s.d. = 4.3) v. 3.2 (s.d. = 3.6). The adjusted treatment effect coefficient was 1.29 (95% CI –1.14 to 3.72, t 36 = 1.04, P = 0.3), indicating that the post-treatment scores did not show a significant difference between treatment and control.
Discussion
Our results support our primary hypothesis that the intervention would reduce the number and duration of bipolar relapses of any type. Significant differences were observed on all three parameters of relapse, indicating that participants in the treatment group were less likely to have a relapse and spent less time unwell. We also observed that the treatment group were less likely to have a relapse of depression and spent less time depressed. No manic episode was observed in the treatment group during the 9-month follow-up period.
Sampling
Advantages of our study include the fact that the participants were drawn from a variety of community sources, and were heterogeneous in terms of demographics and illness profile; comorbidities were common and did not have a significant impact on outcomes, suggesting the intervention has applicability when working with people who have additional comorbidities. Our choice of broad inclusion criteria with few exclusions represents a sacrifice of ‘purity’ of the sample as found in most other trials of this nature, in favour of greater generalisability.
Treatment approach
Using the Collaborative Therapy Framework, Reference Gilbert, Miller, Berk, Ho and Castle22 the intervention was informed by focus groups comprising patients, carers and service providers, and a thorough literature review of existing psychosocial interventions. It shares commonalities with psychoeducation and cognitive–behavioural therapy interventions, and incorporates elements of dialectical behaviour therapy (e.g. certain distress tolerance skills), social rhythms and motivational interviewing. This makes it arguably less ‘pure’ than some other interventions in the field, but this selected multimodal approach has the virtue of being able to apply different established therapeutic techniques and technologies according to the needs of individuals.
Translation into clinical practice
Concern has been raised in a recent meta-analysis about whether the outcomes of interventions developed and conducted by experts in the field will be replicated when conducted by clinicians in everyday clinical practice. Reference Beynon, Soares-Weiser, Woolacott, Duffy and Geddes2 Translating evidence-based interventions into clinical practice is a challenge for all researchers and is relevant but not unique to psychosocial interventions for bipolar disorder. To enhance the process of translation, this study was conducted in partnership with mental health services. By training mental health clinicians from different service settings, across the multidisciplinary team, the potential usefulness of this intervention in everyday clinical practice has been highlighted. Reference Castle and Gilbert17,Reference Gilbert, Miller, Berk, Ho and Castle22
Unlike many available interventions for bipolar disorder, the group format allowed the intervention to be delivered at relatively low cost. Of equal value, participants were able to share their experience of living with bipolar disorder, a direct benefit of the group process itself. This mode of delivery was well tolerated by participants, as evidenced by high rates of retention through all phases of the study.
Limitations
Although this study has shown positive outcomes we would like to acknowledge the following limitations.
Data collection
To obtain the relapse data, monthly telephone interviews were performed by clinicians using a semi-structured interview schedule. These clinicians were not masked to treatment or control condition; however, detailed records of telephone calls were taken and audited by a masked reviewer for fidelity of data collection protocol. The null results observed in the outcome measures of psychiatric symptoms may be due to lack of power to detect differences or represent limitations in the choice of measures themselves. For example, the suitability of the MADRS for assessing bipolar depression has been questioned. Reference Berk, Malhi, Mitchell, Carman, Hadzi-Pavlovic and Hawkins23 It may also reflect the difficulties inherent in conducting clinical research in naturalistic settings where, despite clear research procedures, participants in an episode of illness may decline to attend a follow-up interview or consent to a home visit, preferring to postpone the interview until they are more stable. Thus, our monthly capture of relapse data is seen as a better measure of efficacy than cross-sectional symptom ratings.
Comparison groups
Participants were not masked to allocation to treatment or control condition which may have contributed to expectation bias. Future studies that address this would need to contain a placebo control arm and incorporate masking of both therapist and patient. The lack of a standardised control intervention that takes into account parameters including the group process itself and therapist contact time is a confounder here, as in many comparable studies. Although Colom et al used a befriending control condition, Reference Colom, Vieta, Sánchez-Moreno, Palomino-Otiniano, Reinares and Goikolea3 most studies in this area have used the pragmatic alternative of usual treatment as the control condition. Reference Scott, Colom and Vieta1,Reference Beynon, Soares-Weiser, Woolacott, Duffy and Geddes2 This is an appropriate option where the research question asks whether this intervention makes a difference to relapse rates over and above treatment as usual. The control condition in our study consisted of usual treatment combined with supportive telephone calls to account for therapist contact time. However, the level of support varied between treatment and control group, which might have influenced outcomes.
Analysis
In the analysis every person in the treatment group was treated as independent, which does not account for any clustering effects that might have occurred within and between treatment group cohorts. Controlling for clustering was not explicitly incorporated into the original design of this trial but is worthy of investigation as part of future studies.
Implications for future research
As with other psychosocial interventions that have combined a number of intervention techniques in a comprehensive programme, various mechanisms of action could explain the outcomes. Future research should aim to assess moderators and mediators of outcome, in order to determine which aspects of the intervention are most salient and which patients will respond best to particular interventions. The results of our study highlight the potential benefits of a manualised group-based psychosocial treatment in reducing relapse. Further large-scale trials may replicate these results and confirm the value of this approach in the routine treatment of bipolar disorder.
Appendix
Outline of the intervention
Sessions 1–2: Education
Introduction to bipolar disorder and triggers commonly associated with the disorder
Sessions 3–6: Core skills development
Monitoring and assessment of stress/triggers Preventing relapse using coping strategies, e.g. problem-solving, stress tolerance, SMART goal-setting and medication management
Sessions 7–9: Depression
Assessing and managing prodromes of depression
Developing collaborative relapse prevention plans for depression
Sessions 10–12: Mania and hypomania
Assessing and managing prodromes of mania/hypomania
Developing collaborative relapse prevention plans for mania/hypomania
Booster sessions 1–3: Reinforcing and integrating skills
Consolidating coping strategies into daily life
Funding
The study was funded by the Medical Benefits Fund Foundation and beyondblue Victorian Centre of Excellence in Depression and Related Disorders grants.
Acknowledgements
We are grateful to all those who participated in this project. We acknowledge the contribution of other members of the research team including Cathy Carman, Terence Chong, Catherine Bunton, Kathleen Crowley, Katie Wyman and Brendan Pawsey; the cooperation of our service partners Barwon Health, Pathways Rehabilitation and Support Services Inc, Geelong Mood Support Group, the Geelong Clinic, the Melbourne Clinic and Swinburne Psychology Clinic; and the commitment of service-based clinicians in co-facilitating the group programme and providing us with valuable feedback. We particularly would like to thank the participants for their involvement in this trial.








eLetters
No eLetters have been published for this article.