Abstract
It is not known how immaturity and disease influence postnatal thyroid function in infants <30 wk of gestational age. We performed serial measurements of plasma thyroxine (T4), free T4 (FT4), triiodothyronine (T3), reverse T3 (rT3), TSH, and T4-binding globulin (TBG) in 100 infants of <30 wk of gestation, during the first 8 postnatal weeks, to investigate the influences of disease and gestational age on the time course of thyroid hormones. One hundred infants were divided twice into two groups: 1) in a group of 25-28 and of 28-30 wk of gestation; and 2) in a sick and a healthy group, with similar gestational ages. The time course of T4, FT4, T3, TSH, and TBG, but not rT3 differed significantly (p < 0.005) between the gestational age groups. T4 and FT4 decreased to levels below the cord blood value with a deeper FT4 nadir on d 7 in the youngest group. Disease decreased T4, FT4, T3, TSH, and TBG concentrations especially during the 1st wk after birth (p < 0.005). However, the FT4 nadir on d 7 was similar in sick and healthy infants. After 3 wk, T4, FT4, T3, and TBG were higher in the sick group compared with the healthy group. rT3 levels were not increased in sick infants. We conclude that the extent of the FT4 decrease after birth in infants of <30 wk gestation is mainly influenced by gestational age and probably reflects a transient depletion of thyroidal hormone reserves. rT3 cannot be used as a marker of nonthyroidal illness in very preterm infants.
Similar content being viewed by others
Main
Transient hypothyroxinemia is common in preterm infants. It is more severe the lower the gestational age(1–3). It is arbitrarily defined as a total T4 plasma level of less than 85 nmol/L(2) or 65 nmol/L(3) without a concurrent TSH elevation of more than 20 mU/L. Severe hypothyroxinemia has been related to an increased risk of neurodevelopmental problems(4, 5). However, we found in a randomized trial that T4 supplementation does not change developmental outcome in infants less than 30 wk of gestational age, whereas infants of <27 wk gestation might benefit(6).
To better understand possible clinical implications of transient hypothyroxinemia in very preterm infants, it is important to get more insight into mechanisms behind changes in thyroid hormone levels in the first weeks after birth. Most literature on thyroid function of preterm infants appeared after the introduction of screening programs for congenital hypothyroidism, i.e. after 1976(1) until about 1988(3). Much has changed in the care for very preterm infants since then. Moreover, nowadays a greater proportion of infants of less than 30 wk gestation survives. Recently, two studies were published on T4, FT4, and TSH values of very preterm infants in the neonatal period(7, 8). These studies along with others(3, 9) describe T4 and FT4 values decreasing to values below the cord blood level in the first 7-10 d after birth. However, a complete longitudinal picture of thyroid function of very preterm infants, in which T3, rT3, and TBG are also measured, is still lacking. In addition, in most studies describing disease influences on the time course of T4, the mean gestational age was lower in the sick group than in the healthy group(1, 3), thus complicating interpretation of the results. Changes in thyroid function occur in patients with a variety of nonthyroidal illnesses. In adults, most prominent changes in thyroid function are a decrease in plasma T3 and an increase in plasma rT3(10). It is until now not known how disease changes thyroid function in very preterm infants, and whether disease or low gestational age are the determinative factor for the decrease in T4 shortly after birth.
Therefore, we have analyzed the effect of gestational age (immaturity) and disease on the course of plasma T4, FT4, T3, rT3, TSH, and TBG in the first 8 wk after birth in 100 infants belonging to the placebo group of our randomized trial of T4 supplementation(6). The study was carried out in an iodinereplete region(11, 12)
METHODS
Patient population. The plasma samples analyzed in this study were obtained from 100 infants of the placebo group of a double-blind, randomized, placebo-controlled trial of postnatal T4 supplementation(6). Infants were enrolled in this trial between January 1991 and July 1993. Inclusion criteria were 1) gestational age 25-30 wk, 2) entering into study before the 24th h after birth, 3) absence of severe congenital malformations, 4) no maternal endocrine disease, and 5) no maternal narcotic drug abuse.
Effect of gestational age. To study the gestational age effect on thyroid hormone metabolism, the 100 infants were divided into two groups: a group of infants at <28 wk of gestation and a group of infants at ≥28 wk of gestation. Gestational age was determined from a reliable menstrual history. When maternal information was inconclusive, an early ultrasonography or the Dubowitz score(13) was used. Table 1 presents clinical data of the two gestational age groups.
Effect of disease. To study the effect of disease most effectively, a healthy group (having as little neonatal problems as possible) and a sick group (having severe neonatal problems) were identified who did not differ with regard to gestational age and birth weight. Criteria for the healthy group were: no need for artificial ventilation, no need for surfactant therapy, no proven sepsis, no need for inotropic drugs, no bronchopulmonary dysplasia, less than 10 d on supplemental oxygen, and less than 14 d on total parenteral nutrition. The minimum gestational age of the healthy infants appeared to be 28 wk and 1 d. A sick group was subsequently formed, consisting of infants suffering from severe respiratory distress syndrome (requiring mechanical ventilation and rescue surfactant therapy) with the same minimum gestational age as the healthy group. Thus the sick (n = 14) and the healthy (n = 17) group are part of the gestational age group of infants at ≥28 wk (n = 56). In Table 2 data on the sick and healthy groups are presented.
Respiratory distress syndrome was defined as clinical signs of respiratory distress, the need for supplemental oxygen, and a chest x-ray consistent with respiratory distress syndrome. Surfactant therapy (Curosurf) was given as a rescue therapy according to criteria described earlier(14).
Assays. A blood sample of 1 mL (or less, if the clinical condition did not allow for this quantity) was drawn between 12 and 24 h after birth (d 1) and on d 3, 7, 14, 21, 28, 35, 42, and 56. Cord blood (d 0) was available from 61 infants. Measurements of T4, FT4, T3, rT3, TSH, and TBG were carried out on each of these specimens. The following assays were used: for T4, in-house RIA (detection limit 5 nmol/L; intraassay variation, 3.0%; interassay variation, 5.1%); T3, in-house RIA (detection limit 0.3 nmol/L; intraassay variation, 4.0%; interassay variation, 6.3%); rT3, in-house RIA (detection limit 0.03 nmol/L; intraassay variation, 4.6%; interassay variation, 4.6%); FT4, a two-step RIA assay (SPAC-fT4 fraktion) (BykSangtec Diagnostica, Dietzenbach, Germany) (detection limit, 1.0 pmol/L; intraassay variation, 2.8%; interassay variation, 5.7%); for TSH ICMA (Behring, Amsterdam, the Netherlands) after 10-fold dilution (detection limit 0.01 mU/L, intraassay variation 4.0%, interassay variation 6.0%); and for TBG RIA (Eiken Chemical Co, Tokyo, Japan) (detection limit 20 nmol/L, intraassay variation 6.0%, interassay variation 7.0%.
For invasive procedures 10% diluted povidone was used first, which was rubbed off after 30 s with 70% ethanol in an effort to prevent iodine absorption via the skin(15).
Statistical analysis. Analysis of clinical data. Differences between means were analyzed using the t test (after logarithmic transcription, in case of skewness of the distribution).
Analysis of hormone levels. 1) Effect of gestational age: differences between the mean hormone levels in the gestational age groups were tested by analysis of variance for repeated measurements (BMDP 5V), in which hormone level was examined as a function of time, gestational age (categorized in the two groups) and an interaction term between time and gestational age. 2) Effect of disease: Differences between the mean hormone levels in the “healthy” and “sick” group were tested with the same method, in which hormone level was examined as a function of time, disease and an interaction term between time and disease.
The distribution of residuals was checked for skewness. A log transformation was performed when necessary. All statistical calculations were carried out twice: with surviving infants only and with all infants belonging to the study group (irrespective of survival). The two ways of calculation had comparable results. The results as described here include also infants who died during the study period.
RESULTS
Effect of gestational age. In Figure 1A the time course of plasma T4 concentrations during the first 8 wk after birth is shown for infants of <28 and ≥28 wk gestation. After d 1, T4 decreases to values below the cord blood level in both gestational age groups, with a deeper T4 nadir in infants <28 wk. After d 7, plasma T4 increases until d 28 in both groups. The two curves differed significantly (p = 0.027, interaction term). In Figure 1B FT4 levels are depicted. FT4 levels are lower in the infants <28 wk throughout the whole study period (p = 0.0001), with a deeper nadir on d 7 in infants <28 wk. Also regarding the postnatal T3 course (Fig. 1C), a significant effect of gestational age was found (p = 0.015): At d 3 and 7, T3 levels remain lower in infants <28 wk than in infants ≥28 wk. Thereafter T3 increases steadily in both groups until the end of the 8-wk study period.
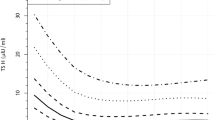
rT3 levels are shown in Figure 1D. No significant difference was found between the two curves. After birth, rT3 decreases steeply until d 7, when rT3 levels stabilize. Although T4, FT4, T3, rT3, and TBG concentrations in cord blood are comparable in infants of <28 and ≥28 wk gestation, cord blood TSH levels are lower in infants of shorter gestational age (Fig. 1E). In both study groups TSH decreases until d 3 and increases after d 7. This increase is larger and longer in duration in infants at <28 wk than in infants at ≥28 wk. On d 28, TSH levels in both study groups have stabilized at about 4 mU/L. Statistical analysis revealed a significantly different time course (p = 0.0008, interaction term).
The TBG time course in infants <28 wk is significantly different from the time course in infants ≥28 wk (p = 0.0028, interaction term; Fig. 1F). On d 7 and 14, TBG levels are lower in the group of infants <28 wk. After d 21, however, the highest TBG levels are found in this group.
Effect of disease. Figure 2A shows mean T4 levels in sick and healthy infants. In healthy infants, a T4 surge is found on d 1. Subsequently, T4 decreases, with a nadir on d 7 and increasing thereafter. In sick infants, however, no first day T4 surge is found; after birth, T4 decreases until d 3. On d 7, T4 levels in the sick and healthy group are comparable. The T4 increase in the sick group continues from d 3 until d 28, when T4 levels have become higher in the sick group. The T4 time course differs significantly between the sick and healthy group (p = 0.00009, interaction term). In Figure 2B, FT4 levels are shown. In contrast to total T4, a FT4 surge in the 1st d after birth is also found in sick infants, although quantitatively less than in healthy infants. FT4 levels are lower in the sick group between d 0 and 21. On d 7, however, the depth of the FT4 nadir is comparable in the sick and healthy groups. After 21 d, FT4 is higher in the sick group (significant difference: p = 0.0054, interaction term). In Figure 2C, T3 levels are depicted. T3 cord blood levels do not differ between the sick and healthy groups. The postnatal T3 surge is higher in the healthy group. T3 levels remain higher in the healthy group until d 21, when T3 levels have become higher in the sick group. The T3 time course is significantly different (interaction term, p = 0.0006) between the two groups.
(A-F) Plasma T4, FT4, T3, rT3, TSH, and TBG concentrations ±SEM during the first 8 wk after birth in sick and healthy infants. Of the 14 sick infants, five died (four on d 3 and one on d 15). The minimum number of blood samples on one time point during the study is eight in the sick group and 16 in the healthy group. Seven (of the nine) surviving infants had bronchopulmonary dysplasia on d 28.
In both sick and healthy infants, a rapid fall in rT3 is found between birth and d 7 (Fig. 2D). Thereafter low rT3 values are found in both groups.
TSH levels are lower in sick infants than in healthy infants in the first 3 d after birth (Fig. 2E). TSH increases from d 3 on in the sick group and from d 7 on in the healthy group. This TSH increase in the healthy group is small (25%, 1 mU of TSH/L); in the sick group the increase is larger (300%, 4 mU of TSH/L). After d 28, TSH levels remain stable in both groups. The two curves differed significantly, but only between d 0 and 35: p = 0.044, interaction term). TBG levels are shown in Figure 2F. In healthy infants TBG levels remain in the range of the cord blood level in the first 8 wk. In sick infants, however, TBG decreases after birth and subsequently increases from d 1 on. TBG levels are higher from d 14 onward in the sick group. The time course of TBG in sick and healthy infants differed significantly (p = 0.0028, interaction term).
All calculations were also done with inclusion of sick infants having less severe respiratory disease. Although differences between healthy and sick infants became less pronounced, the time course of all parameters measured when less sick infants were included was parallel to the time course observed with the here described sickest infants.
DISCUSSION
This is the first study in which postnatal thyroid function of very preterm infants was examined as a function of gestational age and of disease. It shows that the time course of plasma T4, FT4, T3, TSH, and TBG, but not of rT3, differs between infants of 25-28 and 28-30 wk gestation and between sick and healthy infants. In addition, this study shows that the FT4 nadir on d 7 is related only to gestational age and not to disease. T3 concentrations remained above the cord blood level and did not decrease in parallel with T4. Apparently T3 levels are the result of an increase in the availability of type I deiodinase, irrespective of the availability of substrate (T4)(16). In both groups TSH increased in response to low T4 levels on d 7. The pronounced difference in TSH between the two gestational age groups on d 14 and 21 appeared to be related to gestational age and not to disease. This may indicate that the thyroid gland is more sensitive to TSH as gestation proceeds, especially beyond 28 wk. The bioactivity of TSH might also improve with progression of gestation due to differences in TSH glycosylation(17).
TBG concentrations were influenced mainly by disease: in sick infants TBG levels decreased after birth; TBG levels were lower in sick infants in the 1st wk after birth and higher from d 14 onward, compared with healthy infants. Lower values of TBG have been described earlier in infants with respiratory distress syndrome(18). Elevated levels of IL-6 in infants with severe respiratory distress syndrome could have decreased TBG synthesis(19). The subsequent TBG increase probably reflects amelioration of liver function after the respiratory distress syndrome has resolved, which usually happens in the 1st wk of life. This disease-associated TBG time course had an effect on the time course of T4 and also T3 (both are TBG bound): no T4 surge was found in the sick group, whereas the T3 surge was attenuated. T4 and T3 were lower in the 1st wk after birth in the sick group and subsequently, after d 21, higher than in the healthy group. No rT3 increase was found in sick infants. In elder children and adults, an rT3 increase is one of the most prominent features of nonthyroidal illness and is thought to occur due to a decrease in hepatic type I deiodinase activity(10). The overall picture in all (sick and healthy) infants of <30 wk gestation is a fast decrease from birth until d 7, when rT3 levels stabilize. The decreased availability of substrate (T4) and the postnatal disappearance of type III deiodinase in the preterm infant itself(16) and of placental origin(20) probably cause the fast decrease of rT3 in the 1st wk after birth.
Thus, the picture of thyroid function in infants at <30 wk of gestation in the first postnatal week shows decreasing T4, FT4, and rT3 concentrations and slightly increasing T3 concentrations with a TSH increase only from d 7 onward. Thyroidal hormone reserves could be transiently depleted after the preterm neonatal T4 surge and the acute demand for thyroid hormones by peripheral tissues. Prenatal TRH therapy to the mother depressed cord blood FT4 levels in infants born 2-10 d after TRH administration(21). Apparently in these infants also, the immature thyroid needs time to recover from a period of increased T4 secretion. If, however, in infants of <30 wk gestation transplacental passage of thyroid hormones still plays a role(22–24), then the FT4 nadir on d 7 might also be caused by acute withdrawal of maternal thyroid hormone resources. Yet, no information is available with respect to placental passage of thyroid hormones in this (25-30 wk) phase of human pregnancy, and more research needs to be done in this field.
In conclusion, the extent of the postnatal T4 and FT4 decrease in infants of <30 wk gestational age is related to gestational age and probably reflects a transient depletion of thyroidal T4 sources. The duration of the period during which FT4 levels are below the cord blood level is related mainly to disease. rT3 cannot be used as a marker of nonthyroidal illness in very preterm infants.
Abbreviations
- T4:
-
thyroxine
- FT4:
-
free thyroxine
- T3:
-
triiodothyronine
- rT3:
-
reverse triiodothyronine
- TBG:
-
thyroxine-binding globulin
References
Uhrmann S, Marks KH, Maisels MJ, Maisels MJ, Friedman Z, Murray F, Kulin HE, Kaplan M, Utiger RD 1978 Thyroid function in the preterm infant: a longitudinal assessmant. J Pediatr 92: 968–973.
Fisher DA Euthyroid low thyroxine and triiodothyronine states in premature and sick neonates. Pediatr Clin North Am 1990 1297–1312
Mercado M, Yu VYH, Francis I, Szymonowicz W, Gold H 1988 Thyroid function in very preterm infants. Early Hum Dev 16: 131–141.
Den Ouden AL, Kok JH, Verkerk PH, Brand R, Verloove-Vanhorick SP 1996 The relation between neonatal thyroxine levels and neurodevelopmental outcome at age 5 and 9 years in a national cohort of very preterm and/or very low birth weight infants. Pediatr Res 39: 142–145.
Reuss ML, Paneth N, Pinto-Martin JA, Lorenz JM, Susser M 1996 Transient hypothyroxinemia in preterm infants and neurodevelopment at age two years. N Engl J Med 334: 821–827.
Van Wassenaer AG, Kok JH, De Vijlder JJM, Briët JM, Smit BJ, Tamminga P, Van Baar AL, Dekker FW, Vulsma T 1997 Effects of thyroxine supplementation on neurologic development in infants born at less than 30 weeks' gestation. N Engl J Med 336: 21–26.
Frank JE, Faix JE, Hermos RJ, Mullaney DM, Rojan DE, Mitchell ML, Klein RZ 1996 Thyroid function in very low birth weight infants: effects on neonatal hypothyroidism screening. J Pediatr 128: 548–554.
Rooman RP, Du Caju MVL, Op De Beeck L, Doex M, Van Reempts P, Van Acker KJ 1996 Low thyroxinemia occurs in the majority of very preterm newborns. Eur J Pediatr 155: 211–215.
Van Wassenaer AG, Kok JH, Endert E, Vulsma T, De Vijlder JJM 1993 Thyroxine supplementation to infants of less than 30 weeks gestational age does not increase plasma triiodothyronine concentrations. Acta Endocrinol 129: 139–146.
Chopra IJ, Chopra U, Smith SR, Reza M, Solomon DH 1975 Reciprocal changes in serum concentrations of 3,3′,5′-triiodothyronine and 3,5,3′-triidothyronine in systemic illnesses. J Clin Endocrinol Metab 41: 1043–1049.
Delange F, Heidemann P, Bourdoux P, Larsson A, Vigneri R, Klett M, Beckers C, Stubbe P 1986 Regional variations of iodine nutrition and thyroid function during the neonatal period in Europe. Biol Neonate 49: 322–330.
Berghout A, Endert E, Ross A, Hogerzeil HV, Smits NJ, Wiersinga WM 1994 Thyroid function and thyroid size in normal pregnant women living in an iodine replete area. Clin Endocrinol 41: 375–379.
Dubowitz LMS, Dubowitz V, Goldberg C 1970 Clinical assessment of gestational age in newborn infants.. J Pediatr 77: 1–10.
Collaborative European Multicenter Study Group 1992 Randomized European multicenter trial of surfactant replacement therapy for severe neonatal respiratory distress syndrome: single versus multiple doses of curosurf. Pediatrics 89: 13–20.
Smerdely P, Boyages SC, Wu D, Leslie G, John E, Lim A, Waite K, Roberts V, Arnold J, Eastman CJ 1989 Topical iodine-containing antiseptics and neonatal hypothyroidism in very-low-birth weight infants. Lancet 2: 661–664.
Fisher DA, Polk DH, Wu SY 1994 Fetal thyroid metabolism: a pluralistic system. Thyroid 4: 367–371.
Gyves PW, Gesundheit N, Taylor T, Butler JB, Weintraub BD 1987 Changes in thyrotropin (TSH) carbohydrate structure and response to TSH-releasing hormone during postnatal ontogeny: analysis by concanavalin-A chromatography. Endocrinology 121: 133–140.
Brock Jacobson B, Peitersen B, Hummer L 1979 Serum concentrations of thyrotropin, thyroid hormones and thyroid hormone-binding proteins during acute and recovery stages of idiopathic respiratory distress syndrome. Acta Paediatr Scand 68: 257–264.
Bartalena L, Farsetti A, Flink IL, Robbins J 1992 Effects of interleukin-6 on the expression of thyroid hormone-binding protein genes in cultured human hepatoblastoma-derived (Hep G2) cells. Mol Endocrinol 6: 935–942.
Roti E, Fang SL, Green K, Braverman LE, Emerson CH 1983 Inner ring deiodination of thyroxine and 3,5,3′-tiidothyronine by human fetal membranes. Am J Obstet Gynecol 147: 788–792.
Ballard PL, Ballard RA, Creasy RK, Padbory J, Polk DH, Bracken M, Moya FR, Gross I 1992 Plasma thyroid hormones and prolactin in infants and their mothers after prenatal treatment with thyrotropin-releasing hormone. Pediatr Res 32: 673–678.
Vulsma T, Gons MG, de Vijlder JJM 1989 Maternal-fetal transfer of thyroxine in congenital hypothyrodism due to a total organification defect or thyroid agenesis. N Engl J Med 321: 13–16.
Costa A, Arisio R, Benedetto C, Bertino E, Fabris C, Giraudi G, Marozio L, Maulà V, Pagliano M, Testori O, Zoppetti G 1991 Thyroid hormones in tissues from human embryos and fetuses. J Endocrinol Invest 14: 559–568.
Morreale de Escobar G, Calvo R, Obregon MJ, Escobar del Rey F 1990 Contribution of maternal thyroxine to fetal thyroxine pools in normal rats near term. Endocrinology 126: 2762–2767.
Author information
Authors and Affiliations
Additional information
Supported by the N.W.O.-Council for medical research (project number: 900-540-160) and by the Praeventiefonds, The Hague (Grant 28-2051), The Netherlands.
Rights and permissions
About this article
Cite this article
Van Wassenaer, A., Kok, J., Dekker, F. et al. Thyroid Function in Very Preterm Infants: Influences of Gestational Age and Disease. Pediatr Res 42, 604–609 (1997). https://doi.org/10.1203/00006450-199711000-00009
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199711000-00009
This article is cited by
-
Clinical indicators that influence a clinician’s decision to start L-thyroxine treatment in prematurity with transient hypothyroxinemia
Italian Journal of Pediatrics (2023)
-
Effect of levothyroxine supplementation in extremely low birth weight infants with transient hypothyroxinemia of prematurity
Scientific Reports (2022)
-
Effects of oral iodine supplementation in very low birth weight preterm infants for the prevention of thyroid function alterations during the neonatal period: results of a randomised assessor-blinded pilot trial and neurodevelopmental outcomes at 24 months
European Journal of Pediatrics (2022)
-
The factors associated with transient hypothyroxinemia of prematurity
BMC Pediatrics (2021)
-
Initial and delayed thyroid-stimulating hormone elevation in extremely low-birth-weight infants
BMC Pediatrics (2019)