Abstract
An association between chorioamnionitis and periventricular leukomalacia has been reported in human preterm infants. However, whether this link is causal has not been convincingly established, and the underlying molecular mechanisms remain unclear. The objective of this study was to establish a reproducible model of cerebral white matter disease in preterm rabbits after intrauterine infection. Escherichia coli was inoculated into both uterine horns of laparotomized pregnant rabbits when gestation was 80% complete. The fetuses were delivered by cesarean section and killed 12, 24, or 48 h after the inoculation. Programmed cell death in the white matter was evaluated by hematoxylin-eosin-saffron staining and in situ fragmented DNA labeling (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling). In a first group of 14 pregnant rabbits not treated with antibiotics, all fetuses delivered 48 h after inoculation were stillborn, whereas fetuses extracted 12 or 24 h after inoculation were alive. No significant cell death was detected in the live fetuses compared with the control noninfected rabbits. In a second group of five pregnant rabbits treated with ceftriaxone initiated 24 h after the inoculation and continued until cesarean section was performed 48 h after inoculation, 13 fetuses were alive, but all showed evidence of extensive programmed cell death in the white matter by hematoxylin-eosin-saffron staining and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling. White matter damage became histologically detectable only 48 h after inoculation. Three of the 13 brains displayed periventricular white matter cysts mimicking human cystic periventricular leukomalacia. The high reproducibility of white matter damage in our model should permit further studies aimed at unraveling the molecular mechanisms of periventricular leukomalacia.
Similar content being viewed by others
Main
An association between intrauterine infection and cerebral white matter lesions or cerebral palsy in preterm neonates has been suggested by several clinical studies (1–4). Zupan et al. (2) and Perlman et al. (4) reported that the combination of intrauterine infection and premature rupture of the membranes was associated with a high risk (up to 20%) of PVL. The hypothesis put forward is that chorioamnionitis may induce excess production of cytokines with deleterious effects on the brain of preterm infants (5–7). Several studies found high concentrations of proinflammatory cytokines in amniotic fluid (8–10) or cord blood (11, 12) in cases of chorioamnionitis. Increased IL-6 concentrations have been reported in the amniotic fluid (13, 14) and cord blood (15) of preterm infants who later developed PVL, further supporting a link between maternofetal infection, circulating cytokines, and PVL.
Although these epidemiologic data are compelling, a direct link between chorioamnionitis, cytokine production, and white matter damage has not yet been established in humans. Animal models may be useful for testing this hypothesis directly. However, few animal models are directly relevant to human PVL. In the interesting model of endotoxin-mediated leukoencephalopathy in full-term kittens reported by Gilles et al. (16), the cerebral damage occurred during the postnatal period (between 2 and 20 d of life). Using a similar method, Young et al. (17) obtained cerebral damage with marked inflammatory infiltrates in neonatal dogs at an age of 1 to 10 d, which, in terms of brain development, corresponds approximately to 34–40 wk of gestation in humans, raising questions about the relevance of this model to human PVL. More recently, Yoon et al. (18) reported that ascending intrauterine infection with Escherichia coli caused fetal white matter damage in rabbits; these lesions were observed in 6% of live fetuses.
The clinical and experimental data outlined above prompted us to test the hypothesis that cerebral white matter lesions can be obtained by intrauterine infection in a reproducible manner. We chose the rabbit model. Our goal was to produce periventricular white matter lesions in >50% of live fetuses, at a stage of brain development corresponding to that in human preterm infants. If this hypothesis proved correct, this model could be used both to investigate the molecular mechanisms involved in the association between maternofetal infection and white matter lesions and to test neuroprotective strategies.
METHODS
Maternal inoculation.
New Zealand White rabbits with timed pregnancies were inoculated once with E. coli K1 (kindly provided by Dr. Edouard Bingen, Service de Microbiologie, Hôpital Robert Debré, Paris, France; (19) between 24 and 30 d of gestation; (the length of gestation in rabbits is 33 d). After analgesia with intramuscular ketamine (5 mg/kg) and local lidocaine infiltration, a laparotomy was performed. The two uterine horns were identified visually, and each was inoculated with 0.5 mL of an E. coli suspension containing 104 to 106 colony-forming units per milliliter in saline. After inoculation, the animals were returned to their cages, where they had free access to food and water and were subjected to alternating 12-h cycles of light and dark. The experimental protocols were approved by our institutional review boards and complied with the guidelines of the Institut National de la Santé et de la Recherche Médicale (INSERM).
Fetal delivery and definition of intrauterine infection.
Fetal delivery was by C-section, under anesthesia (5 mg/kg ketamine and 1 mg/kg propofol). Immediately after delivery, maternal and live fetus blood was obtained by cardiac puncture and used for microbiologic studies. Fetuses were considered infected if their blood cultures were positive for E. coli. When microbiologic study was negative, placentas were sampled for histologic studies. Intrauterine infection was defined as presence in the chorionic plate or amnion of diffuse neutrophil infiltrates or positive placenta cultures. After blood sampling, a lethal pentobarbital injection was given to each delivered fetus, and the brain was immediately removed and immersed in 10% formaldehyde.
To allow for the possibility that white matter damage may require some time to become detectable by histologic methods, we tried to maximize in utero survival after E. coli inoculation. To this end, we conducted two sets of experiments. In a first group of rabbits (group A), C-section was performed 12, 24, or 48 h after inoculation. The longest fetal postinoculation survival time in this group was 24 h. Based on this finding, we studied another group (group B), in which the dams were given i.v. ceftriaxone therapy (100 mg/kg per day), starting 24 h after inoculation. C-section was performed 48 h after inoculation. The control group was composed of eight noninfected newborns spontaneously delivered at full term (33 d of gestation).
Cell death in the white matter.
After 8 d of fixation in formaldehyde, the fetal brains were cut in three blocks along the rostrocaudal axis (two blocks contained the cerebral hemispheres and the diencephalon, and the third block contained the brain stem and the cerebellum). After paraffin embedding, 5-μm-thick sections were cut from these three blocks. Eight to 10 groups of five adjacent sections were collected from each block. The distance between groups of adjacent sections was 200 μm. In each group of adjacent sections, the first slide was stained with HES. Histopathologic changes considered suggestive of PCD included intense and uniform nuclear staining, chromatin condensation with karyorrhexis, and formation of apoptotic bodies (plasma membrane enclosing cytoplasm, which frequently contained dense basophilic chromatin). To obtain further evidence of PCD, in situ fragmented DNA was labeled using TUNEL on the sections adjacent to those used for HES staining. An in situ cell death detection kit was used as directed (Boehringer Mannheim, Meylan, France). In brief, sections were deparaffinized, treated for 20 min at 37°C with 20 mg/mL proteinase K, and incubated for 2 min on ice with 0.1% Triton X-100. DNA strand breaks were identified by labeling free 3′-OH terminals for 60 min at 37°C with terminal deoxynucleotidyl transferase, using nucleotides labeled with fluorescein. Incorporated nucleotides were detected using an antifluorescein antibody conjugated with alkaline phosphatase, with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt as the substrate. In the present study, no attempt was made to characterize the cells undergoing PCD.
Immunohistochemical staining.
To identify macrophagic infiltrate, the immunohistochemical staining was performed on formalin-fixed, paraffin-embedded brain tissue. The endogenous peroxidase activity was inhibited using 0.5% hydrogen peroxide in methanol for 10 min. Then, a standard biotin-streptavidin peroxidase method with 3,3′ diaminobenzidine tetrachloride as the chromogene, counterstained with hematoxylin, was used. Immunohistochemistry staining for CD68 was performed with antibody (CD68, DAKO Corp, Carpinteria, CA) and revealed with chemMate system (K5001, DAKO Corp).
Brain sections were analyzed by two independent observers unaware of experimental group allocation (A, B, or control).
RESULTS
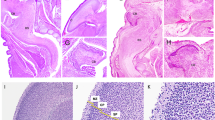
In control rabbits born spontaneously at 32–33 d of gestation, histologic examination did not reveal any detectable brain lesion (Fig. 1A). TUNEL staining showed no labeled cells in most of the studied sections at the level of the periventricular white matter (Fig. 1B); very rare labeled cells were, however, detected in a minority of white matter sections. Few positive cells were TUNEL-positive in the cortical plate, indicative of physiologic PCD occurring at this developmental stage.
In group A, 14 pregnant females were inoculated between 24 and 30 d of gestation (mean, 27.7 d). None of these rabbits received antibiotics. One female spontaneously delivered preterm stillborn fetuses, which were excluded from further analysis. C-section was performed 12, 24, or 48 h after inoculation. Table 1 reports pregnancy outcomes and findings from microbiologic and histologic studies in these three subgroups. All the fetuses delivered 48 h after inoculation were dead; their brains were not studied. When the live fetuses delivered 12 or 24 h after inoculation were compared, no difference in the infection rate was found (79% after 12 h versus 73% after 24 h; not significant by Mantel-Henstel test with Yates correction, p = 0.77). For fetuses delivered at 24 h after inoculation, microbiologic culture of placenta was always positive and therefore histopathologic study was not performed. For the fetuses delivered 12 h after inoculation, microbiologic culture was positive in 35 of 43; histopathologic study of the placenta showed signs of chorioamnionitis in the eight cases with negative culture. None of the live infected fetuses had detectable brain lesion on HES-stained sections. TUNEL revealed a few positive cells in the cortical plate and a very small number of labeled cells scattered throughout the periventricular white matter. The density and distribution of TUNEL-positive cells in these animals were identical to those in controls.
In group B (Table 2), five pregnant females received antibiotic therapy starting 24 h after inoculation. Gestational age at inoculation was 27–28 d (mean, 27.4 d) and was not significantly different from that in group A (not significant by Mann-Whitney U test, p = 0.52). One inoculated female died before C-section and was excluded from the analysis. Forty-eight hours after inoculation, 35 fetuses were delivered from the four surviving mothers. Twenty-two were stillborn. In the 13 live fetuses, histologic examination revealed in 12 brains (92%) multifocal PCD affecting the periventricular white matter, the hippocampus, and the brain stem; focal PCD in the white matter was found in one brain. The TUNEL study showed labeled cells in all 13 brains. Compared with control or group A brains, group B brains had far larger numbers of TUNEL-positive cells in the hippocampus, brain stem, and periventricular white matter, where these cells were grouped in numerous clusters. The density of TUNEL-positive cells in white matter varied somewhat across fetuses but, in a given fetus, was relatively uniform throughout the white matter. The topographic correlation between HES and TUNEL findings was excellent (Fig. 2).
Typical adjacent brain sections at the level of the periventricular white matter from a fetus extracted 48 h after intrauterine E. coli inoculation and treated by maternal antibiotic therapy. A, histologic study revealed PCD with intense, uniform nuclear staining and chromatin condensation (arrowhead). HES staining ×40. B, TUNEL method confirmed PCD with labeled cells grouped in numerous clusters (arrowhead). TUNEL staining ×40.
In most group B fetuses, TUNEL-positive cells were found in moderate numbers in the cortical plate, as in controls. However, three group B fetuses, which displayed widespread white matter, hippocampal, and brain stem PCD, had significantly higher densities of TUNEL-positive cells in the neocortex. Cavitation of periventricular white matter with a macrophagic reaction (Fig. 3A) was observed in these three brains; the macrophagic nature of the infiltrating cells was confirmed by the immunohistochemical staining with CD68 staining (Fig. 3B). No granulocyte infiltrates were found in the brains by HES staining. In the 13 live fetuses, blood cultures were negative, whereas maternal blood cultures were positive. Histologic examination of the chorionic plates and amnions showed focal neutrophil infiltrates (Fig. 4) in all cases, although microbiologic studies were negative.
Adjacent brain sections (A, HES staining ×5;B, anti-CD68 immunolabeling ×5) at the level of the periventricular white matter (PV) from a fetus extracted 48 h after intrauterine E. coli inoculation and treated by maternal antibiotic therapy. Abbreviations:CAV, periventricular white matter cyst;VL, ventricular cavity. Arrowheads indicate examples of CD68-labeled cells, evocative of macrophagic infiltrate.
DISCUSSION
The key finding from our study is that E. coli inoculation into the uterine horns in combination with maternal antibiotic treatment initiated 24 h after inoculation produced intrauterine infection and cerebral white matter PCD in 100% of live fetuses. A 48-h lag time between E. coli inoculation and fetal brain harvesting was needed to observe PCD in the white matter; brain damage was not detectable in fetuses killed at earlier times.
Relevance of the fetal rabbit model to human preterm infants.
Our model replicates several features typical of human preterm infants. The time-courses of rabbit and human brain development are roughly the same: embryogenesis occurs early during gestation, neuronal migration is completed before birth, and myelination peaks during the postnatal period (20). The size of the periventricular white matter is much larger when compared with mice and rats, making the rabbit model quite appropriate to study PVL. Both rabbits and humans have a hemochorial type of placentation, which produces the closest possible contact between the maternal and fetal circulations (21). Moreover, each fetus has its own placenta and the fetus-placenta unit is extremely useful for studying associations between maternal disease states and fetal outcomes. Finally, the financial cost is affordable, and experimental reproducibility is excellent and easily achieved.
Maternofetal infection and white matter disease.
The goal of our study was to obtain direct evidence of a reproducible link between intrauterine infection and cerebral white matter disease. As previously mentioned, epidemiologic studies have shown a statistically significant correlation between chorioamnionitis (2–4), but not neonatal infection (22, 23), and the occurrence of PVL in human preterm babies. In an autopsy study that permitted an estimate of the chronologic onset of PVL, neonatal sepsis was found to be a risk factor only for PVL with postnatal onset (24). Other studies (15, 25) reported high concentrations of circulating and brain cytokines in preterm newborns who later developed PVL, further suggesting that PVL is not related to fetal or neonatal infection but to chorioamnionitis-related production of cytokines (26). Consistent with this hypothesis, our group B animals had a high rate of PCD in the white matter but had negative blood cultures. The absence of fetal infection in group B contrasts with the 73% rate of fetal infection in group A. This difference may be ascribable to eradication of fetal infection by the antimicrobial therapy used in group B. However, available data do not allow us to establish this. To our knowledge, the impact of maternal antibiotic therapy on rabbit fetuses with positive blood cultures has not been investigated. Several studies of ascending infection in pregnant rabbits (27–29) found increased fetal survival after maternal antibiotic therapy but did not provide evidence that the surviving fetuses were infected before antibiotic therapy introduction. In the model developed by McDuffie and Gibbs (28), all amniotic fluid and endometrial cultures were negative in the group given antibiotic therapy immediately after inoculation, whereas 30% and 47% of cultures, respectively, were positive in the untreated group. Similarly, Heddleston et al. (29) reported that maternal antibiotic therapy initiated immediately after inoculation increased fetal survival but also significantly decreased intrauterine infection. The improvement in fetal survival in these studies is probably ascribable to inhibition of intrauterine infection development rather than to eradication of the organism in previously infected fetuses. Thus, the live noninfected fetuses in our group B were probably not yet infected at the time of antibiotic therapy introduction.
The considerably higher rate of PCD in the white matter in our model (100%) compared with the 6% rate in the study by Yoon et al. (18) may be related to differences in experimental design:1) maternal antibiotic therapy was started 30 min after inoculation in the study by Yoon et al. (18) versus 24 h after inoculation in our study; interestingly, this suggests that effective neuroprotection is most likely to be achieved when antibiotics are introduced in the very early stages of chorioamnionitis;2) inoculation was intravaginal in the study by Yoon et al. (18) and intraamniotic in our study; and 3) concentration of the inoculum was 103 to 104 colony-forming units per milliliter in the study by Yoon et al. (18) versus 104 to106 colony-forming units per milliliter in our study.
PCD in the white matter.
White matter cell death required 24–48 h to be detectable by histologic techniques. This delay suggests a PCD, as shown by other models of brain insults (30–33). For example, Renolleau et al. (30) found that the peak of PCD occurred between 24 h and 96 h after focal ischemia in neonatal rats. In our study, the histologic features of dying cells on the HES-stained sections were consistent with PCD, and TUNEL findings further supported PCD. However, studies using other techniques, such as DNA electrophoresis, electron microscopy, and investigation of cell death-related proteins, are required to confirm the PCD in the white matter.
Our neuropathologic findings in the white matter of rabbit fetuses were strikingly similar to those found in human PVL. First, the distribution of dying cells in the periventricular region in rabbit brains matched the distribution of brain lesions in human preterm babies with PVL. Second, three rabbit fetuses displayed periventricular white matter cysts exhibiting several of the features of cystic PVL. Finally, these three brains showed macrophagic activity, a feature of human PVL (34).
The pathophysiologic mechanisms underlying the postchorioamnionitis white matter lesions in our model remain unclear. The negative blood cultures in fetuses with brain lesions and the absence of cerebral granulocyte infiltrates, such as those described in models of experimental neonatal meningitis (35), militate strongly against brain infection and, therefore, against infection as a direct cause of the cerebral damage. Our data suggest an indirect mechanism of brain lesion production, consistent with the hypothesis of cytokine involvement in human PVL (26). For example, TNF-α is a potent inducer of apoptosis (36, 37). Several studies found that high concentrations of circulating TNF-α and other proinflammatory cytokines were associated with chorioamnionitis or PVL (12–15). TNF-α has been shown to increase permeability (5) and to cross the blood-brain barrier (38). Microglial cells and astrocytes are capable of producing TNF-α (39), and microglial expression of TNF-α immunoreactivity was found in the white matter of infants with PVL (25). Although these results suggest that TNF-α and other proinflammatory cytokines may play an important role in perinatal cerebral white matter disease, further studies using our model and other available models (16, 30, 32, 40) are needed to test this hypothesis directly. Such studies should measure cytokine concentrations in maternal and fetal blood as well as cytokine expression in placentas and fetal brains.
In conclusion, we described a rabbit model that reproducibly demonstrated a causal association between intrauterine infection and PCD in the periventricular white matter. The absence of fetal infection in animals with brain lesions supported the hypothesis that the white matter damage was mediated by circulating factors, such as cytokines, produced in response to chorioamnionitis. The high reproducibility of white matter lesions in our study, together with the anatomic and embryologic similarities between rabbits and humans, strongly suggest that our rabbit model may be useful for studying human PVL and its prevention.
Abbreviations
- PVL:
-
periventricular leukomalacia
- PCD:
-
programmed cell death
- C-section:
-
cesarean section
- TUNEL:
-
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- TNF-α:
-
tumor necrosis factor-α
- HES:
-
hematoxylin-eosin-saffron
References
Murphy DJ, Sellers S, MacKenzie IZ, Yudkin PL, Johnson AM 1995 Case-control study of antenatal and intrapartum risk factors for cerebral palsy in very preterm singleton babies. Lancet 346: 1449–1454
Zupan V, Gonzales P, Lacaze-Masmonteil T, Boithias C, d'Allest AM, Dehan M, Gabilan JC 1996 Periventricular leukomalacia: risk factors revisited. Dev Med Child Neurol 38: 1061–1067
Verma U, Tejani N, Klein S, Reale MR, Beneck D, Figueroa R, Visintainer P 1997 Obstetric antecedents of intraventricular hemorrhage and periventricular leukomalacia in the low-birth-weight neonate. Am J Obstet Gynecol 176: 275–281
Perlman JM, Risser R, Broyles RS 1996 Bilateral cystic periventricular leukomalacia in the premature infant: associated risk factors. Pediatrics 97: 822–827
Megyeri P, Abraham CS, Temesvari P, Kovacs J, Vas T, Speer CP 1992 Recombinant human tumor necrosis factor alpha constricts pial arterioles and increases blood-brain barrier permeability in newborn piglets. Neurosci Lett 148: 137–140
Adinolfi M 1993 Infectious disease in pregnancy, cytokines and neurological impairment: an hypothesis. Dev Med Child Neurol 35: 549–553
Leviton A 1993 Preterm birth and cerebral palsy: is tumor necrosis factor the missing link?. Dev Med Child Neurol 35: 553–558
Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA 1993 The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 81: 941–948
Greig PC, Ernest JM, Teot L, Erikson M, Talley R 1993 Amniotic fluid interleukin-6 levels correlate with histologic chorioamnionitis and amniotic fluid cultures in patients in premature labor with intact membranes. Am J Obstet Gynecol 169: 1035–1044
Romero R, Avila C, Santhanam U, Sehgal PB 1990 Amniotic fluid interleukin 6 in preterm labor: association with infection. J Clin Invest 85: 1392–1400
Singh B, Merchant P, Walker CR, Kryworuchko M, Diaz-Mitoma F 1996 Interleukin-6 expression in cord blood of patients with clinical chorioamnionitis. Pediatr Res 39: 976–979
Lencki SG, Maciulla MB, Eglinton GS 1994 Maternal and umbilical cord serum interleukin levels in preterm labor with clinical chorioamnionitis. Am J Obstet Gynecol 170: 1345–1351
Martinez E, Figueroa R, Garry D, Visintainer P, Patel K, Verma U, Sehgal PB, Tejani N 1998 Elevated amniotic fluid interleukin-6 as a predictor of neonatal periventricular leukomalacia and intraventricular hemorrhage. J Matern Fetal Invest 8: 101–107
Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO 1997 Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1β, and tumor necrosis factor-α), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 177: 19–26
Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, Syn HC 1996 Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol 174: 1433–1440
Gilles FH, Leviton A, Kerr CS 1976 Endotoxin leucoencephalopathy in the telencephalon of the newborn kitten. J Neurol Sci 27: 183–191
Young RSK, Yagel SK, Towfighi J 1983 Systemic and neuropathologic effects of E. Pediatr Res 17: 349–353
Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, Chi JG 1997 Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol 177: 797–802
Bingen E, Bonacorsi S, Brahimi N, Denamur E, Elion J 1997 Virulence patterns of Escherichia coli K1 strains associated with neonatal meningitis. J Clin Microbiol 11: 2981–2982
Hoar RM, Monie IW 1981 Comparative development of specific organ systems. In: Kimmel CA, Buelke-Sam J (eds) Developmental Toxicology. Raven Press, New York, pp 13–33
Kozma C, Macklin W, Cummins LM, Mauer R 1974 Anatomy, physiology and biochemistry of the rabbit. In: Weisbroth SH, Flatt RE, Kraus AL (eds) The Biology of the Laboratory Rabbit. Academic Press, New York, pp 50–72
De Vries LS, Regev R, Dubowitz MS, Whitelaw A, Aber VR 1988 Perinatal risk factors for the development of extensive cystic leukomalacia. Am J Dis Child 142: 732–735
Tzogalis D, Fawer CL, Wong Y, Calame A 1988 Risk factors associated with the development of peri-intraventricular haemorrhage and periventricular leukomalacia. Helv Paediat Acta 43: 363–376
Murphy DJ, Squier MV, Hope PL, Sellers S, Johnson A 1996 Clinical associations and time of onset of cerebral white matter damage in very preterm babies. Arch Dis Child 75: F27–F32
Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, Chi JG 1997 High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol 177: 406–411
Dammann O, Leviton A 1997 Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 42: 1–8
McDuffie RS Jr, Blanton SJ, Shikes RH, Gibbs RS 1991 A rabbit model for bacterially induced preterm pregnancy loss: intervention studies with ampicillin-sulbactam. Am J Obstet Gynecol 165: 1568–1574
McDuffie RS Jr, Gibbs RS 1996 Ascending group B streptococcal genital infection in the rabbit model. Am J Obstet Gynecol 175: 402–405
Heddleston L, McDuffie RS Jr, Gibbs RS 1993 A rabbit model for ascending infection in pregnancy: intervention with indomethacin and delayed ampicillin-sulbactam therapy. Am J Obstet Gynecol 169: 708–712
Renolleau S, Aggoun-Zouaoui D, Ben-Ari Y, Charriaut-Marlangue C 1998 A model of transient unilateral focal ischemia with reperfusion in the P7 neonatal rat: morphological changes indicative of apoptosis. Stroke 29: 1454–1460
Pulera MR, Adams LM, Liu H, Santos DG, Nishimura RN, Yang F, Cole GM, Wasterlain CG 1998 Apoptosis in a neonatal rat model of cerebral hypoxia-ischemia. Stroke 29: 2622–2630
Yue X, Mehmet H, Penrice J, Cooper C, Cady E, Wyatt JS, Reynolds EO, Edwards AD, Squier MV 1997 Apoptosis and necrosis in the newborn piglet brain following transient cerebral hypoxia-ischaemia. Neuropathol Appl Neurobiol 23: 16–25
Beilharz EJ, Williams CE, Dragunow M, Sirimanne ES, Gluckman PD 1995 Mechanisms of delayed cell death following hypoxic-ischemic injury in the immature rat: evidence of apoptosis during selective neuronal loss. Brain Res Mol Brain Res 29: 1–14
Banker BQ, Larroche JC 1962 Periventricular leukomalacia of infancy. Arch Neurol 7: 386–410
Kim YS, Sheldon RA, Elliott BR, Liu Q, Ferriero DM, Täuber MG 1995 Brain injury in experimental neonatal meningitidis due to group B streptococci. J Neuropathol Exp Neurol 54: 531–539
Pulliam L, Zhou M, Stubblebine M, Bitler CM 1998 Differential modulation of cell death proteins in human brain cells by tumor necrosis factor alpha and platelet activating factor. J Neurosci Res 54: 530–538
Bogdan I, Leib SL, Bergeron M, Chow L, Tauber MG 1997 Tumor necrosis factor-alpha contributes to apoptosis in hippocampal neurons during experimental group B streptococcal meningitidis. J Infect Dis 176: 693–697
Uno H, Matsuyama T, Akita H, Nishimura H, Sugita M 1997 Induction of tumor necrosis factor-alpha in the mouse hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab 17: 491–499
Gutierrez EG, Banks WA, Kastin AJ 1993 Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol 47: 169–176
Marret S, Mukendi R, Gadisseux JF, Gressens P, Evrard P 1995 Effect of ibotenate on brain development: an excitotoxic mouse model of microgyria and posthypoxic-like lesions. J Neuropathol Exp Neurol 54: 358–370
Acknowledgements
The authors thank Dr. Christian Laboisse (Hôtel Dieu, Nantes) for his help. We also thank Anne-Françoise Miegeville, Edith Chalopin, Catherine Dalibon, Sigrid Parois, and Leslie Schwendimann for their excellent technical assistance.
Author information
Authors and Affiliations
Additional information
Supported by the Institut National de la Santé et de la Recherche Médicale (INSERM).
Rights and permissions
About this article
Cite this article
Debillon, T., Gras-Leguen, C., Vérielle, V. et al. Intrauterine Infection Induces Programmed Cell Death in Rabbit Periventricular White Matter. Pediatr Res 47, 736–742 (2000). https://doi.org/10.1203/00006450-200006000-00009
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200006000-00009
This article is cited by
-
A systematic review of immune-based interventions for perinatal neuroprotection: closing the gap between animal studies and human trials
Journal of Neuroinflammation (2023)
-
Prophylactic inhibition of NF-κB expression in microglia leads to attenuation of hypoxic ischemic injury of the immature brain
Journal of Neuroinflammation (2020)
-
Early neuropathological and neurobehavioral consequences of preterm birth in a rabbit model
Scientific Reports (2019)
-
TNFR1-JNK signaling is the shared pathway of neuroinflammation and neurovascular damage after LPS-sensitized hypoxic-ischemic injury in the immature brain
Journal of Neuroinflammation (2014)
-
High-field diffusion tensor imaging characterization of cerebral white matter injury in lipopolysaccharide-exposed fetal sheep
Pediatric Research (2012)