Abstract
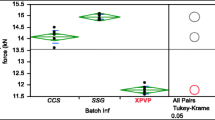
The aims of this study are (1) to compare the disintegration efficiency, and (2) to develop a discriminating test model for the 3 classes of superdisintegrants represented by Ac-Di-Sol, Primojel, and Polyplasdone XL10. Using a digital video camera to examine the disintegration process of tablets containing the same wt/wt percentage concentration of the disintegrants, Ac-Di-Sol was found to disintegrate tablets rapidly into apparently primary particles; Primojel also apparently disintegrated tablets into primary particles but more slowly; Polyplasdone XL10 disintegrated tablets rapidly but into larger masses of aggregated particles. The differences in the size distribution generated in the disintegrated tablets likely contribute to the drug dissolution rate differences found for aspirin tablets with similar disintegration rates. The aspirin tablet matrix is proposed as a model formulation for disintegrant efficiency comparison and performance consistency testing for quality control purposes.
Similar content being viewed by others
References
Bi YX, Sunada H, Yonezawa Y, Danjo K. Evaluation of rapidly disintegrating tablets prepared by a direct compression method.Drug Dev Ind Pharm. 1999;25:571–581.
Sallam E, Ibrahim H, Abu Dahab R, Shubair M, Khalil E. Evaluation of fast disintegrants in terfenadine tablets containing a gas-evolving disintegrant.Drug Dev Ind Pharm. 1998;24:501–507.
Bi Y, Sunada H, Yonezawa Y, et al. Preparation and evaluation of a compressed tablet rapidly disintegrating in the oral cavity.Chem Pharm Bull (Tokyo). 1996;44:2121–2127.
Massimo G, Catellani PL, Santi P, et al. Disintegration propensity of tablets evaluated by means of disintegrating force kinetics.Pharm Dev Technol. 2000;5:163–169.
Caramella C, Colombo P, Conte U, et al. Water uptake and disintegrating force measurements: towards a general understanding of disintegration mechanisms.Drug Dev Ind Pharm. 1986;12:1749–1766.
Johnson JR, Wang LH, Gordon MS, Chowhan ZT. Effect of formulation solubility and hygroscopicity on disintegrant efficiency in tablets prepared by wet granulation, in terms of dissolution.J Pharm. Sci. 1991;80:469–471.
Pandit JK, Tripathi MK, Babu RJ. Effect of tablet disintegrants on the dissolution stability of nalidixic acid tablets.Pharmazie. 1997; 52:538–540.
Sakr A, Bose M, Menon A. Comparative effectiveness of superdisintegrants on the characteristics of directly compressed triamterene hydrochlorothiazide tablets.Pharm Ind. 1993;55:953–957.
Chen CR, Cho SL, Lin CK, Lin YH, Chiang ST, Wu HL. Dissolution difference between acidic and neutral media of acetaminophen tablets containing a super disintegrant and a soluble excipient. II.Chem Pharm Bull (Tokyo). 1998;46:478–481.
Zhao N, Augsburger LL. The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of hydrochlorothiazide from directly compressed tablets.AAPS PharmSciTech. 2005;6:E120-E126.
Gordon MS, Rudraraju VS, Dani K, Chowhan ZT. Effect of the mode of super disintegrant incorporation on dissolution in wet granulated tablets.J Pharm Sci. 1993;82:220–226.
USP.United States Pharmacopeia, 27th revision. Rockville, MD: USP Convention, Inc; 2004:2302.
Rizk S, Barthelemy C, Duru C, Guyot-Herman AM. Investigation on a new modified USP xanthan with tablet-disintegrating properties.Drug Dev Ind Pharm. 1997;23:19–26.
Chang RK, Shinwari M, Leonzio M, Wu LS, Hussain MA. Evaluation of the disintegrant properties for an experimental, crosslinked polyalkylammonium polymer.Int J Pharm. 1998;173:87–92.
Colombo P, Conte U, Caramella C, Geddo M, La Manna A. Disintegrating force as a new formulation parameter.J Pharm Sci. 1984;73:701–705.
Zhao N, Augsburger LL. On the development of a disintegrating functionality test for superdisintegrants.AAPS PharmSci. 2003;5:M1256.
Shah U, Augsburger LL. Evaluation of the functional equivalence of crospovidone NF from different sources. 2. Standard performance test.Pharm Dev Technol. 2001;6:419–430.
Zhao N.Investigation of Super Disintegrant Functionality and Development of Discriminating Test Methods [dissertation]. Baltimore, MD: University of Maryland; 2005.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: December 12, 2005
Rights and permissions
About this article
Cite this article
Zhao, N., Augsburger, L.L. Functionality comparison of 3 classes of superdisintegrants in promoting aspirin tablet disintegration and dissolution. AAPS PharmSciTech 6, 79 (2005). https://doi.org/10.1208/pt060479
Received:
Accepted:
DOI: https://doi.org/10.1208/pt060479