Summary and Conclusions
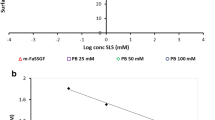
Depending on the dose size and solubility characteristics of low solubility drugs, a meaningful and discriminatory power of dissolution rate testing can be demonstrated. Saturation solubility of fenofibrate and glipizide in different media were determined. Solubility of fenofibrate increased directly with SLS concentration. For a 54-mg fenofibrate tablet, SLS at 0.025 M level is required for a discriminative dissolution test, while for 160-mg tablet, dissolution condition and levels of SLS should be optimized; higher concentrations may be effective (ie, 0.052 M, ∼1.5%). A pH 6.8 phosphate buffer medium is appropriate for glipizide 10-mg tablet dissolution study, when formulation ingredients include excipients with surface activity (eg, HPMC).
Similar content being viewed by others
References
Dressman JB, Amidone GL, Reppas C, Shah VP. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15:11–22.
Pillay V, Fassihi R. Evaluation and comparison of dissolution data derived from different modified release dosage forms: an alternative method. J Control Release. 1998;55:45–55.
Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER). Guidance for Industry. Modified Release Solid Oral Dosage Forms: Scale up and Post Approval Changes (SUPAC): Chemistry, Manufacturing and Controls, In Vitro Dissolution Testing and In Vivo Bioequivalence Documentation. Rockville, MD: FDA; 1997.
Shah VP, Konecny JJ, Everett RL, McCullough B, Noorizadeh AC, Skelly JP. In vitro dissolution profile of water-insoluble drug dosage forms in the presence of surfactants. Pharm Res. 1989;6:612–618.
Abdou HM. Dissolution, Bioavailability, and Bioequivalence. Easton, PA: Mack; 1989.
United States Pharmacopeial Convention. In vitro and in vivo evaluation of dosage forms. USP 28. Rockville, MD: United States Pharmacopeial Convention Inc; 2005:1088.
Carstensen JT. Physico-chemical aspects of drug release. In: Polderman J, ed. Formulation and Preparation of Dosage Forms. Amsterdam, The Netherlands: North-Holland Biomedical Press, Elsevier; 1977.
Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–420.
Jinno J, Oh DM, Crison JR, Amidon GL. Dissolution of water-insoluble drugs: the combined effect of pH and surfactant. J Pharm Sci. 2000;89:268–274.
Jamzad S, Fassihi R. Development of a controlled release low dose class II drug-glipizide. Int J Pharm. 2006;312:24–32.
Durig T, Fassihi R. Evaluation of floating and sticking extended release delivery systems: an unconventional dissolution test. J Control Release. 2000;67:37–44.
Mukerjee P, Mysels KJ. Critical micelle concentrations of aqueous surfactant systems: US department of commerce. NSRDS-NBS. 1971; 36:51.
Amidon GE, Higuchi WI, Ho NF. Theoretical and experimental studies of transport of micelle-solubilized solutes. J Pharm Sci. 1982;71:77–84.
Higuchi WI. Effects of interacting colloids on transport rates. J Pharm Sci. 1964;53:532–535.
Crison JR, Shah VP, Skelly JP, Amidon GL. Drug dissolution into micellar solutions: development of a convective diffusion model and comparison to the film equilibrium model with application to surfactant-facilitated dissolution of carbamazepine. J Pharm Sci. 1996;85:1005–1011.
Balakrishnan A, Rege BD, Amidon GL, Polli JE. Surfactant-mediated dissolution: contributions of solubility enhancement and relatively low micelle diffusivity. J Pharm Sci. 2004;93:2064–2075.
Nerurkar MM, Ho NFH, Burton PS, Vidmar TJ, Borchardt RT. Mechanistic roles of neutral surfactants on concurrent polarized and passive membrane transport of a model peptide in Caco-2 cells. J Pharm Sci. 1997;86:813–821.
Turro NJ, Yekta A. Luminescent probes for detergent solutions, a simple procedure for the determination of the mean aggregation number of micelles. J Am Chem Soc. 1978;100:5951–5952.
Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER). Chemistry review for metaglip tablets. Available at: http://www.fda.gov/eder/foi/nda/2002/21-460_Metaglip_Chemr.pdf. Accessed: September 1, 2005.
Dow Chemical Company. Methocel Cellulose Ethers Technical Handbook. Midland, MI: Dow Chemical Co; 2002.
Tang L, Khan SU, Muhammad NA. Evaluation and selection of bio-relevant dissolution media for a poorly water soluble new chemical entity. Pharm Dev Technol. 2001;6:531–540.
Gu CH, Gandhi RB, Tay LK, Zhou S, Raghavan K. Importance of using physiologically relevant volume of dissolution medium to correlate the oral exposure of formulations of BMS-480188 mesylate. Int J Pharm. 2004;269:195–202.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: April 7, 2006,
Rights and permissions
About this article
Cite this article
Jamzad, S., Fassihi, R. Role of surfactant and pH on dissolution properties of fenofibrate and glipizide—A technical note. AAPS PharmSciTech 7, 33 (2006). https://doi.org/10.1208/pt070233
Received:
Accepted:
DOI: https://doi.org/10.1208/pt070233