ABSTRACT
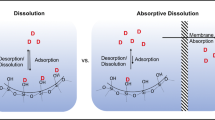
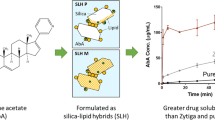
Low dissolution of drugs in the intestinal fluid can limit their effectiveness in oral therapies. Here, a novel porous silica-supported solid lipid system was developed to optimize the oral delivery of drugs with limited aqueous solubility. Using lovastatin (LOV) as the model poorly water-soluble drug, two porous silica-supported solid lipid systems (SSL-A and SSL-S) were fabricated from solid lipid (glyceryl monostearate, GMS) and nanoporous silica particles Aerosil 380 (silica-A) and Syloid 244FP (silica-S) via immersion/solvent evaporation. SSL particles demonstrated significantly higher rate and extent of lipolysis in comparison with the pure solid lipid, depending on the lipid loading levels and the morphology. The highest lipid digestion was observed when silica-S was loaded with 34% (w/w) solid lipid, and differential scanning calorimeter (DSC) analysis confirmed the encapsulation of up to 2% (w/w) non-crystalline LOV in this optimal SSL-S formulation. Drug dissolution under non-digesting intestinal conditions revealed a three- to sixfold increase in dissolution efficiencies when compared to the unformulated drug and a LOV-lipid suspension. Furthermore, the SSL-S provided superior drug solubilization under simulated intestinal digesting condition in comparison with the drug-lipid suspension and drug-loaded silica. Therefore, solid lipid and nanoporous silica provides a synergistic effect on optimizing the solubilization of poorly water-soluble compound and the solid lipid-based porous carrier system provides a promising delivery approach to overcome the oral delivery challenges of poorly water-soluble drugs.








Similar content being viewed by others
References
Amidon G, Lennernäs H, Shah V, Crison J. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6(3):231–48.
Porter CJH, Pouton CW, Cuine JF, Charman WN. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv Drug Deliv Rev. 2008;60(6):673–91.
Trevaskis NL, Charman WN, Porter CJH. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 2008;60(6):702–16.
Zhu Y, D’Agostino J, Zhang Q-Y. Role of intestinal cytochrome P450 (P450) in modulating the bioavailability of oral lovastatin: insights from studies on the intestinal epithelium-specific P450 reductase knockout mouse. Drug Metab Dispos. 2011;39(6):939–43.
Chen CC, Tsai TH, Huang ZR, Fang JY. Effects of lipophilic emulsifiers on the oral administration of lovastatin from nanostructured lipid carriers: physicochemical characterization and pharmacokinetics. Eur J Pharm Biopharm. 2010;74(3):474–82.
Ge Z, Zhang X-x, Gan L, Gan Y. Redispersible, dry emulsion of lovastatin protects against intestinal metabolism and improves bioavailability. Acta Pharmacol Sin. 2008;29(8):990–7.
Mandal S. Microemulsion drug delivery system: design and development for oral bioavailability enhancement of lovastatin. SA Pharm J. 2011;78(3):44–50.
Yanamandra S, Venkatesan N, Kadajji VG, Wang Z, Issar M, Betageri GV. Proliposomes as a drug delivery system to decrease the hepatic first-pass metabolism: case study using a model drug. Eur J Pharm Sci. 2014;64:26–36.
Goyal U, Arora R, Aggarwal G. Formulation design and evaluation of a self-microemulsifying drug delivery system of lovastatin. Acta Pharma. 2012;62(3):357–70.
Suresh G, Manjunath K, Venkateswarlu V, Satyanarayana V. Preparation, characterization, and in vitro and in vivo evaluation of lovastatin solid lipid nanoparticles. AAPS Pharm Sci Technol. 2007;8(1):E162–70.
Seenivasan A, Panda T, Théodore T. Lovastatin nanoparticle synthesis and characterization for better drug delivery. Open Biotechnol J. 2011;5(1):28–32.
Tiwari R, Pathak K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm. 2011;415(1–2):232–43.
Xu W, Riikonen J, Lehto V-P. Mesoporous systems for poorly soluble drugs. Int J Pharm. 2013;453(1):181–97.
Vallet-Regí M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew Chem Int Ed. 2007;46(40):7548–58.
Wu C, Wang J, Hu Y, Zhi Z, Jiang T, Zhang J, et al. Development of a novel starch-derived porous silica monolith for enhancing the dissolution rate of poorly water soluble drug. Mater Sci Eng C. 2012;32(2):201–6.
Simovic S, Heard P, Hui H, Song Y, Peddie F, Davey AK, et al. Dry hybrid lipid–silica microcapsules engineered from submicron lipid droplets and nanoparticles as a novel delivery system for poorly soluble drugs. Mol Pharm. 2009;6(3):861–72.
Tan A, Simovic S, Davey AK, Rades T, Prestidge CA. Silica-lipid hybrid (SLH) microcapsules: a novel oral delivery system for poorly soluble drugs. J Control Release. 2009;134(1):62–70.
Yasmin R, Tan A, Bremmell KE, Prestidge CA. Lyophilized silica lipid hybrid (SLH) carriers for poorly water-soluble drugs: physicochemical and in vitro pharmaceutical investigations. J Pharm Sci. 2014;103(9):2950–9.
Bremmell KE, Tan A, Martin A, Prestidge CA. Tableting lipid-based formulations for oral drug delivery: a case study with silica nanoparticle–lipid–mannitol hybrid microparticles. J Pharm Sci. 2013;102(2):684–93.
Joyce P, Tan A, Whitby CP, Prestidge CA. The role of porous nanostructure in controlling lipase-mediated digestion of lipid loaded into silica particles. Langmuir. 2014;30(10):2779–88.
Simovic S, Hui H, Song Y, Davey AK, Rades T, Prestidge CA. An oral delivery system for indomethacin engineered from cationic lipid emulsions and silica nanoparticles. J Control Release. 2010;143(3):367–73.
Rao S, Tan A, Boyd BJ, Prestidge CA. Synergistic role of self-emulsifying lipids and nanostructured porous silica particles in optimizing the oral delivery of lovastatin. Nanomedicine. 2014;1–15.
Porter CJH, Charman WN. Uptake of drugs into the intestinal lymphatics after oral administration. Adv Drug Deliv Rev. 1997;25(1):71–89.
Venishetty VK, Chede R, Komuravelli R, Adepu L, Sistla R, Diwan PV. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: a novel strategy to avoid intraduodenal administration. Colloids Surf B: Biointerfaces. 2012;95:1–9.
Müller RH, Shegokar R, Keck CM. 20 years of lipid nanoparticles (SLN & NLC): present state of development & industrial applications. Curr Drug Discov Technol. 2011;8(3):207–27.
Porter CJH, Kaukonen AM, Taillardat-Bertschinger A, Boyd BJ, O’Connor JM, Edwards GA, et al. Use of in vitro lipid digestion data to explain the in vivo performance of triglyceride-based oral lipid formulations of poorly water-soluble drugs: studies with halofantrine. J Pharm Sci. 2004;93(5):1110–21.
Sek L, Porter CJH, Kaukonen AM, Charman WN. Evaluation of the in-vitro digestion profiles of long and medium chain glycerides and the phase behaviour of their lipolytic products. J Pharm Pharmacol. 2002;54(1):29–41.
The Dissolution Procedure: Development and Validation [database on the Internet]. 2013 [cited 22 Nov. 2013]. Available from: http://www.usp.org/usp-nf/notices/general-chapter-dissolution-procedure-development-and-validation.
Reis P, Holmberg K, Watzke H, Leser ME, Miller R. Lipases at interfaces: a review. Adv Colloid Interf Sci. 2009;147–148:237–50.
Wickham M, Garrood M, Leney J, Wilson PDG, Fillery-Travis A. Modification of a phospholipid stabilized emulsion interface by bile salt: effect on pancreatic lipase activity. J Lipid Res. 1998;39(3):623–32.
Li Y, Hu M, McClements DJ. Factors affecting lipase digestibility of emulsified lipids using an in vitro digestion model: proposal for a standardised pH-stat method. Food Chem. 2011;126(2):498–505.
Hanefeld U, Gardossi L, Magner E. Understanding enzyme immobilisation. Chem Soc Rev. 2009;38(2):453–68.
Jiang T, Wu C, Gao Y, Zhu W, Wan L, Wang Z, et al. Preparation of novel porous starch microsphere foam for loading and release of poorly water soluble drug. Drug Dev Ind Pharm. 2014;40(2):252–9.
Vallet-Regi M, Doadrio JC, Doadrio AL, Izquierdo-Barba I, Pérez-Pariente J. Hexagonal ordered mesoporous material as a matrix for the controlled release of amoxicillin. Solid State Ionics. 2004;172(1–4):435–9.
Aerts CA, Verraedt E, Depla A, Follens L, Froyen L, Van Humbeeck J, et al. Potential of amorphous microporous silica for ibuprofen controlled release. Int J Pharm. 2010;397(1–2):84–91.
Siegel RA, Rathbone MJ, editors. Overview of controlled release mechanisms. Fundamentals and applications of controlled release drug delivery. United States: Springer; 2012. p. 19–46.
Santamaría E, Maestro A, Porras M, Gutiérrez JM, González C. Controlled release of ibuprofen by meso-macroporous silica. J Solid State Chem. 2014;210:242–50.
Larsen AT, Åkesson P, Juréus A, Saaby L, Abu-Rmaileh R, Abrahamsson B, et al. Bioavailability of cinnarizine in dogs: effect of SNEDDS loading level and correlation with cinnarizine solubilization during in vitro lipolysis. Pharm Res. 2013;30(12):3101–13.
Christophersen PC, Christiansen ML, Holm R, Kristensen J, Jacobsen J, Abrahamsson B, et al. Fed and fasted state gastro-intestinal in vitro lipolysis: in vitro in vivo relations of a conventional tablet, a SNEDDS and a solidified SNEDDS. Eur J Pharm Sci. 2014;57:232–9.
Tan A, Rao S, Prestidge CA. Transforming lipid-based oral drug delivery systems into solid dosage forms: an overview of solid carriers, physicochemical properties, and biopharmaceutical performance. Pharm Res 2013;1–25
Neusilin. Fuji Chemical Industries. Available from: http://www.neusilin.com/product/general_properties.php.
Acknowledgments
This work has been supported by the Australian Research Council (ARC) under DP120101065. The University of South Australia and Ian Wark Research Institute are acknowledged for the scholarship funded to Rokhsana Yasmin. Also Mr. Achal Bhatt and Mr. Vaskor Bala are acknowledged for advice and assistance during manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Otilia M. Koo, Panayiotis P. Constantinides, Lavinia M. Lewis, and Joseph Reo
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table I
(DOCX 373 kb)
Supplementary Table II
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Yasmin, R., Rao, S., Bremmell, K.E. et al. Porous Silica-Supported Solid Lipid Particles for Enhanced Solubilization of Poorly Soluble Drugs. AAPS J 18, 876–885 (2016). https://doi.org/10.1208/s12248-015-9864-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-015-9864-z