Abstract
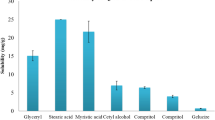
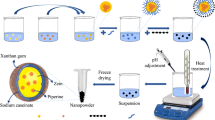
The main objective of the present study was to investigate the influence of various formulation parameters on the preparation of zein nanoparticles. 6,7-dihydroxycoumarin (DHC) was used as a model hydrophobic compound. The influence of pH of the aqueous phase, buffer type, ionic strength, surfactant, and zein concentration on particle size, polydispersity index, and zeta potential of DHC-loaded zein nanoparticles were studied. Smaller nanoparticles were formed when the pH was close to the isoelectric point of zein. DHC-loaded zein nanoparticles prepared using citrate buffer (pH 7.4) was better than phosphate buffer in preventing particle aggregation during lyophilization. The ionic strength did not have a significant influence on the particle size of DHC-loaded zein nanoparticles. A combination of Pluronic F68 and lecithin in 2:1 ratio stabilized the zein nanoparticles. An increase in zein concentration led to increase in particle size of DHC-loaded zein nanoparticles. The use of optimal conditions produced DHC-loaded nanoparticles of 256 ± 30 nm and an encapsulation efficiency of 78 ± 7%. Overall, the study demonstrated the optimal conditions to prepare zein nanoparticles for drug encapsulation.









Similar content being viewed by others
REFERENCES
Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41:2545–61.
Wang G, Uldag H. Recent developments in nanoparticle-based drug delivery and targeting systems with emphasis on protein-based nanoparticles. Exp Opin Drug Deliv. 2008;5:499–515.
Langer K, Balthasar S, Vogel V, Dinauer N, Briesen HV, Shubert D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int J Pharm. 2003;257:169–80.
Kaul G, Amiji M. Tumor-targeted gene delivery using poly (ethylene glycol)-modified gelatin nanoparticles: in vitro and in vivo studies. Pharm Res. 2005;22:951–61.
Reddy N, Yang Y. Potential of plant proteins for medical applications. Trend Biotechnol. 2011;29:490–8.
Satheesh P, Radhey K, Omathanu P. Protein based nanoparticulate drug delivery systems. In: Yashwant P, Deepak T, editors. Nanoparticulate drug delivery systems. II. Formulation and characterization. New York: Informa Healthcare; 2009. p. 69–91.
Yi YM, Yang TY, Pan WM. Preparation and distribution of 5-fluorouracil (125)I sodium alginate bovine serum albumin nanoparticles. World J Gastrol. 1999;5:57–60.
Cascone MG, Lazzeri L, Carmignani C, Zhu Z. Gelatin nanoparticles produced by a simple emulsion as delivery system for methotrexate. J Mater Sci Mater Med. 2002;13:523–6.
Wang SZ, Esen A. Primary structure of a proline rich zein and its cDNA. Plant Physiol. 1986;81:70–4.
Shukla R, Cheryan M. Zein: the industrial protein from corn. Ind Crops Prod. 2001;13:171–92.
Moros EE, Darnoko D, Cheryan M, Perkins EG, Jerrell J. Analysis of xanthophylls in corn by HPLC. J Agric Food Chem. 2002;50:5787–90.
Kale A, Zhu F, Cheryan M. Separation of high-value products from ethanol extracts of corn by chromatography. Ind Crop Prod. 2007;26:44–53.
Anonymous. Wheat gluten, corn gluten and zein film: affirmation of GRAS status. Fed Regist. 1985;50:8997–8999.
Gong SJ, Sun SX, Sun QS, Wang JY, Liu XM, Liu GY. Tablets based on compressed zein microspheres for sustained oral administration: design, pharmacokinetics and clinical study. J Biomater Appl. 2011;26:195–208.
Wang HJ, Lin ZX, Liu XM, Sheng SY, Wang JY. Heparin-loaded zein microsphere film and hemocompatibility. J Control Release. 2005;105:120–31.
Lai LF, Guo HX. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Int J Pharm. 2011;404:317–23.
Luo Y, Teng Z, Wang Q. Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3. J Agric Food Chem. 2012;60:836–43.
Patel A, Hu Y, Tiwari JK, Velikov KP. Synthesis and characterization of zein-curcumin colloidal particles. Soft Matter. 2010;6:6192–9.
Hurtando-Lopez P, Murdan S. Formulation and characterization of zein microspheres as drug delivery vehicles. J Drug Deliv Sci Tech. 2005;15:267–72.
Zhong Q, Jin M. Zein nanoparticles produced by liquid–liquid dispersion. Food Hydrocolloid. 2009;23:2380–7.
Zhong Q, Jin M, Xiao D, Tian H, Zhang W. Application of supercritical anti-solvent technologies for the synthesis of delivery systems of bioactive components. Food Biophys. 2008;3:186–90.
Satheesh P, Perumal O. Preparation of zein nanoparticles by pH controlled nanoprecipitation method. J Biomed Nanotechnol. 2010;6:312–7.
Guo Y, Liu Z, An H, Li M, Hu J. Nanostructure and properties of maize zein studied by atomic force microscopy. J Cereal Sci. 2005;41:277–81.
Demchak RJ, Dybas RA. Photostability of abamectin/zein microspheres. J Agric Food Chem. 1997;45:260–2.
Patel AR, Elisabeth CM, Bouwens M, Velikov KP. Sodium caseinate stabilized zein colloidal particles. J Agric Food Chem. 2010;58:12497–503.
Anhorn MG, Mahler HC, Langer K. Freeze drying of human serum albumin (HAS) nanoparticles with different excipients. Int J Pharm. 2008;363:162–9.
Magoshi J, Nakamura S, Murakami K. Structure and physical properties of seed proteins. I. Glass transition and crystallization of zein protein from corn. J Appl Polym Sci. 1992;45:2043–8.
Langer K, Anhorn MG, Steinhauser I, Dreis S, Celebi D, Schrickel N, et al. Human serum albumin (HSA) nanoparticles: reproducibility of preparation process and kinetics of enzymatic degradation. Int J Pharm. 2008;347:109–17.
Esen A. Separation of alcohol-soluble proteins (zeins) from maize into three fractions by differential solubility. Plant Physiol. 1986;80:623–7.
Irache JM, Bergougnoux L, Ezpeleta I, Gueguen J, Orecchioni AM. Optimization and in vitro stability of legumin nanoparticles obtained by a coacervation method. Int J Pharm. 1995;126:103–9.
Cabra V, Arreguin R, Vazquez-Duhalt R, Farres A. Effect of temperature and pH on the secondary structure and processes of oligomerization of 19 kDa alpha-zein. Biochim Biophys Acta. 2006;1764:1110–8.
Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv Drug Deliv Rev. 2006;58:1688–713.
Shalaev EY, Johnson-Elton TD, Chang L, Pikal MJ. Thermophysical properties of pharmaceutically compatible buffers at sub-zero temperatures: implications for freeze-drying. Pharm Res. 2002;19:195–201.
Reddy N, Li Y, Yang Y. Alkali-catalyzed low temperatures wet cross linking of plant proteins using carboxylic acids. Biotechnol Prog. 2009;25:139–46.
Duclairoir C, Nakache E, Marchais H, Orecchioni AM. Formation of gliadin nanoparticles: influence of the solubility parameter of the protein solvent. Colloid Polym Sci. 1998;276:321–7.
Fairhurst D, Lee RW. The zeta potential and its use in pharmaceutical applications—part 1: charged interfaces in polar and non-polar media and the concept of zeta potential. Drug Dev Deliv. 2011;11:60–4.
Schubert MA, Muller-Goymann CC. Characterization of surface modified solid lipid nanoparticles (SLN): influence of lecithin and non-ionic emulsifier. Eur J Pharm Biopharm. 2000;61:77–86.
Vanessa CFM, Philippe L, Huguette PA, Francis P, Gillian B. Poly(d,l-lactide) nanocapsules prepared by a solvent displacement process: influence of the composition on physicochemical and structural properties. J Pharm Sci. 2000;89:614–26.
Dulieu C, Bazile D. Influence of lipid nanocapsules composition on their aptness to freeze-drying. Pharm Res. 2005;22:285–92.
Hong YZ, Xing T, Hong YL, Xiao LL. A lipid microsphere vehicle for vinorelbine: stability, and safety and pharmacokinetics. Int J Pharm. 2008;348:70–9.
Dulclairoir C, Nakache E. Polymer nanoparticle characterization in aqueous suspensions. Int J Polym Anal Charac. 2002;7:284–313.
ACKNOWLEDGMENTS
This work was supported by the South Dakota State Corn Utilization Council, Governor’s 2010 competitive research seed grant, and Department of Pharmaceutical Sciences, South Dakota State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Podaralla, S., Perumal, O. Influence of Formulation Factors on the Preparation of Zein Nanoparticles. AAPS PharmSciTech 13, 919–927 (2012). https://doi.org/10.1208/s12249-012-9816-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-012-9816-1