Abstract
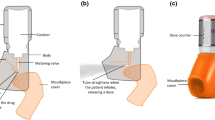
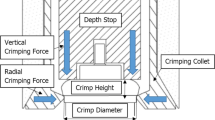
Pressurized metered dose inhalers (MDIs) were first introduced in the 1950s and they are currently widely prescribed as portable systems to treat pulmonary conditions. MDIs consist of a formulation containing dissolved or suspended drug and hardware needed to contain the formulation and enable efficient and consistent dose delivery to the patient. The device hardware includes a canister that is appropriately sized to contain sufficient formulation for the required number of doses, a metering valve capable of delivering a consistent amount of drug with each dose delivered, an actuator mouthpiece that atomizes the formulation and serves as a conduit to deliver the aerosol to the patient, and often an indicating mechanism that provides information to the patient on the number of doses remaining. This review focuses on the current state-of-the-art of MDI hardware and includes discussion of enhancements made to the device’s core subsystems. In addition, technologies that aid the correct use of MDIs will be discussed. These include spacers, valved holding chambers, and breath-actuated devices. Many of the improvements discussed in this article increase the ability of MDI systems to meet regulatory specifications. Innovations that enhance the functionality of MDIs continue to be balanced by the fact that a key advantage of MDI systems is their low cost per dose. The expansion of the health care market in developing countries and the increased focus on health care costs in many developed countries will ensure that MDIs remain a cost-effective crucial delivery system for treating pulmonary conditions for many years to come.









Similar content being viewed by others
References
Lavorini F, Corrigan C, Barnes P, Dekhuijzen P, Levy M, Pedersen S, et al. Retail sales of inhalation devices in European countries: so much for a global policy. Respir Med. 2011;105(7):1099–103.
Doan Q, Shefrin A, Johnson D. Cost-effectiveness of metered-dose inhalers for asthma exacerbations in the pediatric emergency department. Pediatrics. 2011;127(5):e1105–11.
Thiel CG. From Susie’s question to CFC free: an inventor’s perspective on forty years of MDI development and regulation. Respir Drug Deliv. 1996;1:115–24.
Price D, Bosnic-Anticevich S, Briggs A, Chrystyn H, Rand C, Scheuch G, et al. Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med. 2012;107(1):37–46.
Ross DL, Gabrio BJ. Advances in metered dose inhaler technology with the development of a chlorofluorocarbon-free drug delivery system. J Aerosol Med. 1999;12(3):151–60.
Fradley G, Hodson D. Optimization of fluid flow in pMDI valves. Respir Drug Deliv. 2008;2:329–32.
Colombani AC, Chambers F, Lee K, Jansen R, Hodson D. Understanding the cause of increasing dose through life from a pressurised metered dose inhaler (pMDI). J Pharm Pharmacol. 2010;62(10):1314–5.
Knecht A, Daab O, Weil H, inventors; Boehringer Ingelheim KG, assignee. Sediment baffle for valves of pressurized containers. United States patent US 4,944,433. 1990 July 31.
Hodson PD, inventor; Minnesota Mining and Manufacturing Company, assignee. Aerosol dispenser. World Intellectual Property Organization patent WO 1999/036334. 1999 July 20.
Bryant AM, Miller NC, Hodson PD, inventors; Minnesota Mining and Manufacturing Company, assignee. Free flow aerosol valves. United States patent US 5,772,085. 1998 June 30.
Howlett D, Conroy G, inventors; Bespak plc, assignee. Metering valve for dispensers. European patent EP 801,009. 2001 Nov 7.
Hodson PD, inventor; 3M Innovative Properties Company, assignee. Metering valve for a metered dose inhaler providing consistent delivery. World Intellectual Property Organization patent WO 2004/022142. 2004 Feb 26.
Greenleaf DJ, Hodson PD, Purkins G, Mahon GD, Klein HG. Metering valve for a metered dose inhaler providing consistent delivery. World Intellectual Property Organization patent WO 2004/022143. 2004 March 18.
Jinks PA, inventor; Minnesota Mining and Manufacturing Company, assignee. Aerosol valves with movable agitator. United States patent US 5,593,069. 1997 Jan 14.
Brambilla G, Lewis DA, Johnson R, Howlett D, inventors; Chiesi Farmaceutici S.p.A., assignee. Metered-dose inhaler and method of using the same. World Intellectual Property Organization patent WO 2012/230,010. 2012, Mar 15.
Stein SW, Forsyth BR, Stefely JS, Christensen JD, Alband TD, Jinks PA. Expanding the dosing range of metered dose inhalers through formulation and hardware optimization. Respir Drug Deliv. 2004;1:125–34.
Alston W, Clark AR, Schuler C, Walsh KR, inventors; Alston W, Clark AR, Nektar Therapeutics, Schuler C, Walsh KR, assignees. Increased dosage metered dose inhaler. World Intellectual Property Organization patent WO 2004/041340. 2004 May 31.
Lewis DA, O’Shea H, Johnson R, Church T. Predicting HFA-MDI dose retention properties: engineering the marriage between canisters, valves and formulations. Respir Drug Deliv Eur. 2011;1:89–100.
Marecki PE, inventor; Minnesota Mining and Manufacturing Company, assignee. Device for delivering an aerosol. United States patent US 6,006,745. 1999 Dec 28.
Vervaet C, Byron PR. Drug–surfactant–propellant interactions in HFA-formulations. Int J Pharm. 1999;186(1):13–30.
Jinks PA. A new high performance dual-layer coating for inhalation hardware. Conference proceedings of Drug Delivery to the Lungs, 18. 2008.
Dohmeier DM, Heyworth D, Wilde T. The application of a new high performance dual-layer coating to pressurized metered dose inhaler hardware. Respir Drug Deliv Eur. 2009;2:209–12.
Boardman LR, Johnson PR, Korba GA, Mueller ME, Pellerite MJ, Ravichandran R, inventors; 3M Innovative Properties Company, assignee. Medicinal formulation container with a treated metal surface. World Intellectual Property Organization patent WO 2007/112312. 2007 Oct 4.
Fallon JK, Peyron ID, Hickey AJ. Effects of direct spiking of silicone oil into a model pMDI formulation. Drug Dev Ind Pharm. 2013;39(5):681–6.
Sherwood JK, Alex S, Salama G, Obenauer-Kutner L, Huyck S, Berry J, et al. Particle size coarsening induced by valve silicone in a metered dose inhaler. Drug Dev Ind Pharm. 2007;33(2):155–62.
Berry J, Kline L, Naini V, Chaudhry S, Hart J, Sequeira J. Influence of the valve lubricant on the aerodynamic particle size of a metered dose inhaler. Drug Dev Ind Pharm. 2004;30(3):267–75.
Rehfuss BD, Alband TD, Schultz DW, McIntyre DR, Wu Z. Formulation of a current asthma therapy in a non-CFC pMDI propellant system [abstract]. American Assoc Pharm Sci. Annual Meeting, Indianapolis, USA. 2000.
Wu Z, Thatcher ML, Lundberg JK, Ogawa MK, Jacoby CB, Battiste JL, et al. Forced degradation studies of corticosteroids with an alumina–steroid–ethanol model for predicting chemical stability and degradation products of pressurized metered‐dose inhaler formulations. J Pharm Sci. 2012;101(6):2109–22.
Ashurst IC, Herman CS, Li-Bovet L, Riehe MT, inventors; Glaxo Wellcome Inc, Glaxo Group Limited, assignees. Metered dose inhaler for albuterol. United States patent US 6,131,566. 2000 Oct 17.
Lewis D, Ganderton D, Meakin B, Ventura P, Brambilla G, Garzia R, inventors; Chiesi Farmaceutici S.P.A., assignee. Pressurized metered dose inhalers (MDI). World Intellectual Property Organization patent WO 2000/030608. 2000 Jun 2.
Wu ZZ, Govind N, Johnson PR, inventors; 3M Innovative Properties Company, assignee. Steroid solution aerosol products with enhanced chemical stability. European patent EP 1,693,053. 2011 Sept 7.
Lewis D, Ganderton D, Meakin B, Ventura P, Brambilla G, Garzia R, inventors; Chiesi Farmaceutici S.p.A., assignee. Pressurized metered dose inhalers (MDI) containing a solution comprising ipratropium bromide, HFA propellant, and co-solvent and comprising a container with a specific internal surface composition and/or lining. United States patent US 8,142,763. 2012 Mar 27.
Brewis D, Burns S, Colthorpe P, inventors; AstraZeneca, assignee. Surface modification process. World Intellectual Property Organization patent WO 2002/47829. 2002 Jun 20.
Stevenson P, Bromley-Davenport D, inventors; Portal Medical Ltd., assignee. Method of manufacturing a medicament dispenser device. United States patent US 19,863. 2013 Jan 24.
Clayton CD, Scott JS, inventors; 3M Innovative Properties Company, assignee. Medicinal aerosols. World Intellectual Property Organization patent WO 2002/056949. 2002 Jul 25.
Moore JM, Bradley L, Charnock P, Brown S. Container closure system solutions for delivering low numbers of doses from a pressurised metered dose inhaler. Respir Drug Deliv. 2004;2:333–6.
Kellerman DJ, Armer TA, Borland SW, Nelson TS. Dihydroergotamine inhalation aerosol for the treatment of migraine: insights from pharmaceutical and clinical development. Respir Drug Deliv Eur. 2013;1:35–46.
Clark AR. Metered atomisation for respiratory drug delivery [Ph.D. thesis]. Loughboro: Loughborough University; 1991.
Dunbar C, Watkins A, Miller J. An experimental investigation of the spray issued from a pMDI using laser diagnostic techniques. J Aerosol Med. 1997;10(4):351–68.
Dunbar C, Miller J. Theoretical investigation of the spray from a pressurized metered-dose inhaler. Atomization Sprays. 1997;7(4):417–36.
Gabrio BJ, Stein SW, Velasquez DJ. A new method to evaluate plume characteristics of hydrofluoroalkane and chlorofluorocarbon metered dose inhalers. Int J Pharm. 1999;186(1):3–12.
Dunbar CA, Hickey AJ. pMDI optimization using an actuator flow model. Respir Drug Deliv. 1998;1:319–22.
Stein SW, Myrdal PB. The relationship between MDI droplet lifetime and drug delivery efficiency. Respir Drug Deliv. 2006;2:351–6.
Berry J, Heimbecher S, Hart JL, Sequeira J. Influence of the metering chamber volume and actuator design on the aerodynamic particle size of a metered dose inhaler. Drug Dev Ind Pharm. 2003;29(8):865–76.
Stein SW, Myrdal PB. A theoretical and experimental analysis of formulation and device parameters affecting solution MDI size distributions. J Pharm Sci. 2004;93(8):2158–75.
Lewis DA, Meakin BJ, Brambilla G. New actuators versus old: reasons and results for actuator modifications for HFA solution MDIs. Respir Drug Deliv. 2006;1:101–10.
Lewis DA, Ganderton D, Meakin BJ, Brambilla G. Theory and practice with solution systems. Respir Drug Deliv. 2004;1:109–16.
Purewal TS. Formulation of metered dose inhalers. In: Purewal TS, Grant DJW, editors. Metered dose inhaler technology. 1st ed. Illinois: Interpharm; 1998. p. 6–68.
Genova PA, Williams III RC, Jewett W, inventors; IEP Pharmaceutical Devices Inc., assignee. Low spray force, low retention atomization system. United States patent US 6,418,925. 2002 Jul 16.
Versteeg HK, Hargrave GK. Fundamentals and resilience of the original MDI actuator design. Respir Drug Deliv. 2006;1:91–100.
Kakade PP, Versteeg HK, Hargrave GK, Genova P, Williams Iii RC, Deaton D. Design optimization of a novel pMDI actuator for systemic drug delivery. J Aerosol Med. 2007;20(4):460–74.
Lefebvre AH. Atomization and sprays. Washington, D.C.: CRC; 1989.
Blower AW, Clarke MJ, Corbett JS, inventors; Fisons plc, assignee. Aerosol device having a spout to prevent spray head blockage. United States patent US 5,682,875. 1997 Nov 4.
Fachinger J, Hoelz H, Spallek MW, Ziegler J. Nozzle design characterization of metered dose inhalers (MDI) using quality by design (QbD) tools. Respir Drug Deliv Eur. 2009;2:261–4.
Chen Y, Traini D, Fletcher D, Chan H, Lewis D, Church T, et al. The effect of pressurized metered dose inhaler (pMDI) actuator nozzle design on triboelectrification and aerosol deposition. Respir Drug Deliv Eur 2013;2:439–44.
Stein SW, Myrdal PB. The relative influence of atomization and evaporation on metered dose inhaler drug delivery efficiency. Aerosol Sci Tech. 2006;40(5):335–47.
Ju D, Shrimpton J, Bowdrey M, Hearn A. Effect of expansion chamber geometry on atomization and spray dispersion characters of a flashing mixture containing inerts. Part I. Numerical predictions and dual laser measurements. Int J Pharm. 2012;432(1):23–31.
Ju D, Shrimpton J, Bowdrey M, Hearn A. Effect of expansion chamber geometry on atomization and spray dispersion characters of a flashing mixture containing inerts. Part II: high speed imaging measurements. Int J Pharm. 2012;432(1):32–41.
Dunbar C. Atomization mechanisms of the pressurized metered dose inhaler. Part Sci Technol. 1997;15(3):195–216.
Stein SW, Gabrio BJ. Understanding throat deposition during cascade impactor testing. Respir Drug Deliv. 2000;2:287–90.
Lin T, Breysse PN, Laube BL, Swift DL. Mouthpiece diameter affects deposition efficiency in cast models of the human oral airways. J Aerosol Med. 2001;14(3):335–41.
Armer TA, Evans BB, Mohsen NM, Pavkov RM, Sudhalkar AM, inventors; Sheffield Pharmaceuticals Inc., assignee. Methods and apparatus for delivering aerosolized medication. United States patent US 6,095,141. 2000 Aug 1.
Gabrio BJ, Velasquez DJ, inventors; 3M Innovative Properties Company, assignee. Slow spray metered dose inhaler. United States patent US 6,615,826. 2003 Sep 9.
Newman S, Clarke S. Bronchodilator delivery from Gentlehaler, a new low-velocity pressurized aerosol inhaler. Chest. 1993;103(5):1442–6.
Howlett D, inventor; Bespak plc, assignee. Inhaler for medicament. United States patent US 6,062,214. 2000 May 16.
Shrewsbury SB, Armer TA, Newman SP, Pitcairn G. Breath-synchronized plume-control inhaler for pulmonary delivery of fluticasone propionate. Int J Pharm. 2008;356(1):137–43.
Mohsen NM. pMDI aerosol momentum manipulation and biotargeted delivery. Respir Drug Deliv. 2002;2:593–8.
Crompton GK. Problems patients have using pressurized aerosol inhalers. Eur J Respir Dis Suppl. 1982;119:101–4.
Larsen JS, Hahn M, Ekholm B, Wick KA. Evaluation of conventional press-and-breathe metered-dose inhaler technique in 501 patients. J Asthma. 1994;31(3):193–9.
Newman SP, Weisz A, Talaee N, Clarke S. Improvement of drug delivery with a breath actuated pressurised aerosol for patients with poor inhaler technique. Thorax. 1991;46(10):712–6.
Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ. Influence of particle size and patient dosing technique on lung deposition of HFA-beclomethasone from a metered dose inhaler. J Aerosol Med. 2005;18(4):379–85.
Price DB, Pearce L, Powell SR, Shirley J, Sayers MK. Handling and acceptability of the Easi-Breathe device compared with a conventional metered dose inhaler by patients and practice nurses. Int J Clin Pract. 1999;53(1):31–6.
Hampson NB, Mueller MP. Reduction in patient timing errors using a breath-activated metered dose inhaler. Chest. 1994;106(2):462–5.
Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377(9770):1032–45.
Lenney J, Innes J, Crompton G. Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. Respir Med. 2000;94(5):496–500.
MacMichael DBA, Hearne DJ, inventors; PA Knowledge Limited, assignee. Inhaler mechanism. United States patent US 6,405,727. 2002 Jun 18.
Burge S, Isaacs WP, Smith SJ, Tuckwell JD, inventors; Cambridge Consultants Limited, assignee. Pressurized inhalers. World Intellectual Property Organization patent WO 2004/041339. 2004 Jun 2.
Wakefield K Genova PA, inventors; IEP Pharmaceutical Devices Inc., assignee. Pneumatic breath actuated inhaler. United States patent US 6,328,035. 2001 Dec 11.
Hauser SG, inventor; KOS Pharmaceuticals Inc., assignee. Breath coordinated inhaler. United States patent US 6,155,251. 2000 Dec 5.
Bacon RJ, McDerment I, Bell J. A new breath operated actuator. Respir Drug Deliv. 2002;2:403–6.
Julius SM, Sherman JM, Hendeles L. Accuracy of three electronic monitors for metered-dose inhalers. Chest. 2002;121(3):871–6.
Hickey AJ, Dunbar CA. A new millennium for inhaler technology. Pharm Technol. 1997;21(6):116–25.
Dave R. Inhaling cannabinoids [abstract]. New Horizons in Inhaled Drug Del., Mgmt Forum. London, UK, 2002.
Dolovich MB. Proceedings of Spacer Device Symposium. Drug Information Association; 1995.
Newman SP. Additional technology for pressurized metered dose inhalers. In: Newman SP, editor. Respiratory drug delivery: essential theory & practice. Richmond: Respiratory Drug Delivery Online; 2009. p. 217–56.
Brown PH, Blundell G, Greening AP, Crompton G. Do large volume spacer devices reduce the systemic effects of high dose inhaled corticosteroids? Thorax. 1990;45(10):736–9.
Farrer M, Francis A, Pearce S. Morning serum cortisol concentrations after 2 mg inhaled beclomethasone dipropionate in normal subjects: effect of a 750 ml spacing device. Thorax. 1990;45(10):740–2.
Barry P, O’Callaghan C. The effect of delay, multiple actuations and spacer static charge on the in vitro delivery of budesonide from the Nebuhaler. Br J Clin Pharmacol. 1995;40(1):76–8.
Oliveira RF, Teixeira SF, Silva LF, Teixeira JC, Antunes H. Development of new spacer device geometry: a CFD study (part I). Comput Methods Biomech Biomed Engin. 2012;15(8):825–33.
Bisgaard H, Anhøj J, Wildhaber JH. Spacer devices. In: Bisgaard H, O’Callaghan C, Smaldone GC, editors. Drug delivery to the lung. New York: Marcel Dekker; 2002. p. 389–420.
Ogren R, Baldwin J, Simon R. How patients determine when to replace their metered-dose inhalers. Ann Allergy Asthma Immunol. 1995;75(6):485–9.
Given J, Taveras H, Iverson H, Lepore M. Prospective, open-label assessment of albuterol sulfate hydrofluoroalkane metered-dose inhaler with new integrated dose counter. Allergy Asthma Proc. 2013;34:42–51.
Holt S, Holt A, Weatherall M, Masoli M, Beasley R. Metered dose inhalers: a need for dose counters. Respirology. 2005;10(1):105–6.
Koerner SJ, inventor. Inhaler device with means for assessing its depletion level. United States patent US 5,785,048. 1998 Jul 28.
Hodson PD, Smith DK, inventors; Minnesota Mining and Manufacturing Company, assignee. Aerosol dispenser comprising contents indicator. World Intellectual Property Organization patent WO 1996/009229. 1996 Mar 28.
Mitchell JP, Scarrot PM, Nagel MW, Wiersema KJ, Bates SA, Lusty ME. Performance of a new top-mounted dose indicator for use with pressurized metered dose inhalers. 3rd Triennial World Asthma Meeting. Chicago; 2001.
US Food and Drug Administration. Guidance for industry. Integration of dose-counting mechanisms into MDI drug products. 2003.
Bradshaw DR. Developing dose counters: an appraisal based on regulator, pharma, and user needs. Respir Drug Deliv. 2006;1:121–32.
Wasserman RL, Sheth K, Lincourt WR, Locantore NW, Rosenweig JC, Crim C. Real-world assessment of a metered-dose inhaler with integrated dose counter. Allergy Asthma Proc. 2006;27(6):486–92.
Sheth K, Wasserman R, Lincourt W, Locantore N, Carranza-Rosenzweig J, Crim C. Fluticasone propionate/salmeterol hydrofluoroalkane via metered‐dose inhaler with integrated dose counter: performance and patient satisfaction. Int J Clin Pract. 2006;60(10):1218–24.
LaForce C, Weinstein C, Nathan RA, Weinstein SF, Staudinger H, Meltzer EO. Patient satisfaction with a pressurized metered-dose inhaler with an integrated dose counter containing a fixed-dose mometasone furoate/formoterol combination. J Asthma. 2011;48(6):625–31.
Weinstein C, Staudinger H, Scott I, Amar NJ, LaForce C. Dose counter performance of mometasone furoate/formoterol inhalers in subjects with asthma or COPD. Respir Med. 2011;105(7):979–88.
Wilby M, Purkins G. The 3M™ integrated dose by dose counter for pMDIs [abstract]. Drug Del to the Lungs, 17. 2006.
Wass ACL, Law BR, inventors. Minnesota Mining and Manufacturing Company, assignee. Aerosol dispenser comprising an indicator assembly. United States patent US 5,349,945. 1994 Sep 27.
Marx E, inventor; 3M Innovative Properties Company, assignee. Dose indicators and dispensing canister-indicator assemblies. European patent EP 1,369,139. 2003 Dec 10.
Rand PK, Brand PJ, Godfrey JW, Bonney SG, inventors; Glaxo Group Limited, assignee. Dispenser with doses’ counter. World Intellectual Property Organization patent WO 1998/056444. 1998 Dec 17.
Jewett W, Ebeling FA, inventors; IEP Group Inc., assignee. Counter for fluid dispensers. United States patent US 5,622,163. 1997 Apr 22.
Jewett W, Ebeling FA, inventors; IEP Group Inc., assignee. Metered dose inhalator. United States patent US 5,544,647. 1996 Aug 13.
Patel M, Pilcher J, Chan A, Perrin K, Black P, Beasley R. Six-month in vitro validation of a metered-dose inhaler electronic monitoring device: implications for asthma clinical trial use. J Allergy Clin Immunol. 2012;130:1420–2.
Ozone Secretariat, United Nations Environment Program. The Montreal Protocol on Substances that Deplete the Ozone Layer. 1987.
Graf P. Adverse effects of benzalkonium chloride on the nasal mucosa: allergic rhinitis and rhinitis medicamentosa. Clin Ther. 1999;21(10):1749–55.
Ho C, Wu M, Lan M, Tan C, Yang A. In vitro effects of preservatives in nasal sprays on human nasal epithelial cells. Am J Rhinol. 2008;22(2):125–9.
Mallants R, Jorissen M, Augustijns P. Effect of preservatives on ciliary beat frequency in human nasal epithelial cell culture: single versus multiple exposure. Int J Pharm. 2007;338:64–9.
Acknowledgments
The authors would like to thank Georgina Fradley and Adam Stuart (both of 3M Healthcare Ltd., Loughborough, UK) for their assistance with the generation of the figures in this article.
Conflict of Interest
Stephen W. Stein is currently employed by 3M Drug Delivery Systems. P. David Hodson is currently employed by 3M Healthcare Limited. Poonam Sheth and Paul B. Myrdal declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Paul B. Myrdal and Stephen W. Stein
Rights and permissions
About this article
Cite this article
Stein, S.W., Sheth, P., Hodson, P.D. et al. Advances in Metered Dose Inhaler Technology: Hardware Development. AAPS PharmSciTech 15, 326–338 (2014). https://doi.org/10.1208/s12249-013-0062-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-013-0062-y