ABSTRACT
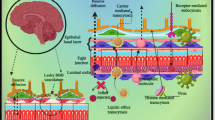
Cerebral tissues possess highly selective and dynamic protection known as blood brain barrier (BBB) that regulates brain homeostasis and provides protection against invading pathogens and various chemicals including drug molecules. Such natural protection strictly monitors entry of drug molecules often required for the management of several diseases and disorders including cerebral vascular and neurological disorders. However, in recent times, the ischemic cerebrovascular disease and clinical manifestation of acute arterial thrombosis are the most common causes of mortality and morbidity worldwide. The management of cerebral Ischemia requires immediate infusion of external thrombolytic into systemic circulation and must cross the blood brain barrier. The major challenge with available thrombolytic is their poor affinity towards the blood brain barrier and cerebral tissue subsequently. In the clinical practice, a high dose of thrombolytic often prescribed to deliver drugs across the blood brain barrier which results in drug dependent toxicity leading to damage of neuronal tissues. In recent times, more emphasis was given to utilize blood brain barrier transport mechanism to deliver drugs in neuronal tissue. The blood brain barrier expresses a series of receptor on membrane became an ideal target for selective drug delivery. In this review, the author has given more emphasis molecular biology of receptor on blood brain barrier and their potential as a carrier for drug molecules to cerebral tissues. Further, the use of nanoscale design and real-time monitoring for developed therapeutic to encounter drug dependent toxicity has been reviewed in this study.




Similar content being viewed by others
REFERENCES
Abbott NJ. Physiology of the blood–brain barrier and its consequences for drug transport to the brain. Int Congr Ser. 2005;1277:3–18.
Jones AR, Shusta EV. Blood–brain barrier transport of therapeutics via receptor mediation. Pharm Res. 2007;24:1759–71.
Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13.
Cardoso FL, Brites D, Brito MA. Looking at the blood–brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64:328–63.
Pardridge WM. Blood–brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3:90–105.
Eyal S, Hsiao P, Unadkat JD. Drug interactions at the blood–brain barrier: fact or fantasy? Pharmacol Ther. 2009;123:80–104.
Shilo M, Motiei M, Hana P, Popovtzer R. Transport of nanoparticles through the blood–brain barrier for imaging and therapeutic applications. Nanoscale. 2014;6(4):2146–52.
Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85.
Ji H, Wang L, Bi H, Sun L, Cai B, Wang Y, et al. Mechanisms of lumbrokinase in protection of cerebral ischemia. Eur J Pharmacol. 2008;590:281–9.
Furlan AJ, Abou-Chebi A. The role of recombinant pro-urokinase (r-pro-UK) and intraarterial thrombolysis in acute ischemic stroke: the PROACT trials. Prolyse in acute cerebral thromboembolism. Curr Med Res Opin. 2002;18 Suppl 2:44–7.
Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129(3):307–21.
Huai Q, Mazar A, Kuo A, Parry GC, Shaw DE, Callahan J, et al. Structure of human urokinase plasminogen activator in complex with its receptor. Science. 2006;311:656–9.
Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97.
Edwards MT, Murphy MM, Geraghty JJ, Wulf JA, Konzen JP. Intraarterial cerebral thrombolysis for acute ischemic stroke in a community hospital. AJNR Am J Neuroradiol. 1999;20(9):1682–7.
Wu JH, Diamond SL. Tissue plasminogen activator (t-PA) inhibits plasmin degradation of fibrin. A mechanism that slows t-PA-mediated fibrinolysis but does not require alpha 2-antiplasmin or leakage of intrinsic plasminogen. J Clin Invest. 1995;95(6):2483–90.
Rhim T, Lee DY, Lee M. Drug delivery systems for the treatment of ischemic stroke. Pharm Res. 2013;30(10):2429–44.
Abbott NJ. Prediction of blood–brain barrier permeation in drug discovery from in vivo, in vitro and in silico models. Drug Discov Today Technol. 2004;1:407–16.
Mruk DD, Su L, Cheng CY. Emerging role for drug transporters at the blood brain barrier. Trends Pharmacol Sci. 2010;32:99–106.
Palmer AM. The role of the blood-CNS barrier in CNS disorders and their treatment. Neurobiol Dis. 2010;37:3–12.
Pardridge WM. Introduction to the blood–brain barrier: methodology, biology, and pathology. Cambridge University PRESS. 1998; 24(9):521–81.
Pardridge WM. Blood–brain barrier delivery. Drug Discov Today. 2007;12:54–61.
Anstrom JA, Brown WR, Moody DM, Thore CR, Challa VR, Block SM. Anatomical analysis of the developing cerebral vasculature in premature neonates: absence of precapillary arteriole to-venous shunts. Pediatr Res. 2002;52:554–60.
Angelow S, Yu AS. Claudins and paracellular transport: an update. Curr Opin Nephrol Hypertens. 2007;16:459–64.
Findley MK, Koval M. Regulation and roles for claudin-family tight junction proteins. IUBMB Life. 2009;61(4):431–7.
Abbott NJ, Ronnback L, Hansson E. Astrocyte–endothelial interactions at the blood brain barrier. Nat Rev Neurosci. 2006;7:41–53.
Pardridge WM. Molecular biology of the blood–brain barrier. Mol Biotechnol. 2005;30:57–70.
Asotra K, Ningaraj N, Black KL. Measurement of blood–brain and blood-tumor barrier permeabilities with (14C)-labeled tracers. Methods Mol Med. 2003;89:177–90.
Pardridge WM. The blood–brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14.
Abulrob A, Sprong H, Bergen Henegouwen PV, Stanimirovic D. The blood–brain barrier transmigrating single domain antibody: mechanisms of transport and antigenic epitopes in human brain endothelial cells. J Neurochem. 2005;95:1201–14.
Schlossauer B, Steuer H. Comparative anatomy, physiology and in vitro models of the blood–brain and blood-retina barrier. Curr Med Chem. 2002;2:175–86.
Ferguson CS, Tyndale RF. Cytochrome P450 enzymes in the brain: emerging evidence of biological significance. Trends Pharmacol Sci. 2011;32:708–14.
Miksys S, Tyndale RF. Brain drug-metabolizing cytochrome P450 enzymes are active in vivo, demonstrated by mechanism-based enzyme inhibition. Neuropsychopharmacology. 2009;34(3):634–40.
Born J, Lange T, Kern W, Mc-Gregor GP, Bickel U, Fehm HL. Sniffing neuropeptides a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–6.
Huang CC, Lee CC, Hsu KS. The role of insulin receptor signalling in synaptic plasticity and cognitive function. Chang Gung Med J. 2010;33(2):115–25.
Zhang Y, Pardridge WM. Rapid transferrin efflux from brain to blood across the blood–brain barrier. J Neurochem. 2001;76:1597–600.
Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC. The blood–brain barrier: an engineering perspective. Front Neuroeng. 2013;6:1–22.
Moos T, Morgan EH. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol. 2001;20:77–95.
Allen DD, Lockman PR, Roder KE, Dwoskin LP, Crooks PA. Active transport of high-affinity choline and nicotine analogs into the central nervous system by the blood–brain barrier choline transporter. J Pharmacol Exp Ther. 2003;304:1268–74.
Smith QR. A review of blood brain barrier transport techniques. Methods Mol Med. 2003;89:193–208.
Hawkins RA, O’Kane RL, Simpson IA, Viña JR. Structure of the blood–brain barrier and its role in the transport of amino acids. J Nutr. 2006;136(1):218S–26.
Zhao F-Q, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8:113–28.
Augustin R, Carayannopoules MO, Dowd LO, Phay JE, Moley JF, Moley KH. Identification and characterization of human glucose transporter-like protein-9 (GLUT9). J Biol Chem. 2004;279:16229–36.
Rip J, Schenk GJ, Boer AG. Differential receptor-mediated drug targeting to the diseased brain. Expert Opin Drug Deliv. 2009;6:227–37.
Gynther M, Laine K, Ropponen J, Leppanen J, Mannila A, Nevalainen T, et al. Large neutral amino acid transporter enables brain drug delivery via prodrugs. J Med Chem. 2008;51(4):932–6.
Peura L, Malmioja K, Huttunen K, Leppanen J, Hamalainen M, Forsberg MM, et al. Design, synthesis and brain uptake of LAT1-targeted amino acid prodrugs of dopamine. Pharm Res. 2013;30(10):2523–37.
Peura L, Malmioja K, Laine K, Leppanen J, Gynther M, Isotalo A, et al. Large amino acid transporter 1 (LAT1) prodrugs of valproic acid: new prodrug design ideas for central nervous system delivery. Mol Pharm. 2011;8(5):1857–66.
Xiao G, Gan L-S. Receptor-mediated endocytosis and brain delivery of therapeutic biologics. Int J Cell Biol. 2013;2013, 703545. 14 pages.
Zhang Y, Pardridge WM. Delivery of β-galactosidase to mouse brain via the blood–brain barrier transferrin receptor. J Pharmacol Exp Ther. 2005;313:1075–81.
Temsamani J, Rousselle C, Rees AR, Scherrmann JM. Vector-mediated drug delivery to the brain. Exp Opin Biol Ther. 2001;1:773–82.
Maresh GA, Maness LM, Zadina JE, Kastin AJ. In vitro demonstration of a saturable transport system for leptin across the blood–brain barrier. Life Sci. 2001;69(1):67–73.
Qian ZM, Li H, Sun H, Ho K. Targeted drug delivery via the transferrin receptor mediated endocytosis pathway. Pharmacol Rev. 2002;54(4):561–87.
Li H, Qian ZM. Transferrin/transferrin receptor-mediated drug delivery. Med Res Rev. 2002;22(3):225–50.
Waheed A, Grubb JH, Zhou XY, Tomatsu S, Fleming RE, Costaldi ME, et al. Regulation of transferrin-mediated iron uptake by HFE, the protein defective in hereditary hemochromatosis. Proc Natl Acad Sci. 2002;99(5):3117–22.
Karsten U, Telli H, Elisabeth H, Jorg K. Transferrin- and transferrin-receptor antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur J Pharm Biopharm. 2009;71(2):251–6.
Van Hoof D, Rodenburg KW, Van der Horst DJ. Receptor-mediated endocytosis and intracellular trafficking of lipoproteins and transferrin in insect cells. Insect Biochem Mol Biol. 2005;35(2):117–28.
Boado RJ, Zhang Y, Zhang Y, Pardridge WM. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood–brain barrier. Biotechnol Bioeng. 2007;96(2):381–91.
Coloma MJ, Lee HJ, Landaw EM, Boado RJ, Morrison SL, Pardridge WM. Transport across the primate blood–brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm Res. 2000;17(3):266–74.
Boado RJ, Zhang Y, Zhang Y, Wang Y, Pardridge WM. GDNF fusion protein for targeted-drug delivery across the human blood–brain barrier. Biotech Bioeng. 2007;100:387–96.
Chung NS, Wasan KM. Potential role of the low-density lipoprotein receptor family as mediators of cellular drug uptake. Adv Drug Deliv Rev. 2004;56(9):1315–34.
Demeule M, Currie JC, Bertrand Y, Che C, Regina A, Gabathuler R, et al. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem. 2008;106(4):1534–44.
May P, Woldt E, Matz RL, Boucher P. The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann Med. 2007;39(3):219–28.
Yoon IS, Chen E, Busse T, Repetto E, Lakshmana MK, Koo EH, et al. Low-density lipoprotein receptor-related protein promotes amyloid precursor protein trafficking to lipid rafts in the endocytic pathway. FASEB J. 2007;21:2742–52.
Harris-White ME, Frautschy SA. Low density lipoprotein receptor-related proteins (LRPs), Alzheimer’s and cognition. Curr Drug Targets CNS Neurol Disord. 2005;4(5):469–80.
Gaillard PJ, Brink A, Boer AG. Diphtheria toxin receptor-targeted brain drug delivery. Int Congr Ser. 2005;1277:185–95.
Bu G, Maksymovitch EA, Nerbonne JM, Schwartz AL. Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neuron. J Biochem. 1994;269:18521–8.
Raab G, Klagsbrun M. Heparin-binding EGF like growth factor. Biochim Biophys Acta. 1997;1333:F179–99.
Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (t-PA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4(2):228–31.
Chernyshev OY, Martin-Schild S, Albright KC, Barreto A, Misra V, Acosta Grotta JC, et al. Safety of t-PA in stroke mimics and neuroimaging-negative cerebral ischemia. Neurology. 2010;74(17):1340–5.
Hatcher MA, Starr JA. Role of tissue plasminogen activator in acute ischemic stroke. Ann Pharmacother. 2011;45(3):364–71.
Cronin CA. Intravenous tissue plasminogen activator for stroke: a review of the ECASS III results in relation to prior clinical trials. J Emerg Med. 2010;38(1):99–105.
Nakashima T, Minematsu K. Prospects of thrombolytic therapy for acute ischemic stroke. Brain Nerve. 2009;61(9):1003–12.
Anderson JL. Development and evaluation of anisoylated plasminogen streptokinase activator complex (APSAC) as a second generation thrombolytic agent. J Am Coll Cardiol. 1987;10(5 Suppl B):22B–7.
Monk JP, Heel RC. Anisoylated plasminogen streptokinase activator complex (APSAC). A review of its mechanism of action, clinical pharmacology and therapeutic use in acute myocardial infarction. Drugs. 1987;34(1):25–49.
Collen D. Staphylokinase: a potent, uniquely fibrin-selective thrombolytic agent. Nat Med. 1998;4(3):279–84.
Moreadith RW, Collen D. Clinical development of PEGylated recombinant staphylokinase (PEG-Sak) for bolus thrombolytic treatment of patients with acute myocardial infarction. Adv Drug Deliv Rev. 2003;55(10):1337–45.
Cooper EL, Hrzenjak TM, Grdisa T. Alternative sources of fibrinolytic, anticoagulative, antimicrobial and anticancer molecules. Int J Immunopathol Pharmacol. 2004;17:237–44.
Wang F, Wang C, Li M, Gui L, Zhang J, Chang W. Purification, characterization and crystallization of a group of earthworm fibrinolytic enzymes from Eisenia fetida. Biotechnol Lett. 2003;25:1105–9.
Inoue T, Yaguchi I, Takayangi K, Hayashi T, Moorrks S, Eguchi Y. A new thrombolytic agent, monteplase, is independent of plasminogen activator inhibitor in patients with acute myocardial infarction: comparison with native t-PA E 610. J Am Coll Cardiol. 1997;29:1447–53.
Sun HL, Jiao JD, Pan ZW, Dong DL, Yang FB. The cardio-protective effect and mechanism of lumbrokinase. Yao Xue Xue Bao. 2006;41(3):247–51.
Justicia C, Gabriel C, Planas AM. Activation of the JAK/STAT pathway following transient focal cerebral ischemia: signaling through Jak1 and Stat3 in astrocytes. Glia. 2000;30:253–70.
Katoh M, Karasawa T, Doi H, Odawara A, Takagi M, Ikeo T, et al. Antiplatelet mechanisms of TA-993 and its metabolite MB3 in ADP-induced platelet aggregation. Eur J Pharmacol. 2000;399:91–6.
Mascareno E, Dhar M, Siddiqui MA. Signal transduction and activator of transcription (STAT) protein-dependent activation of angiotensinogen promoter: a cellular signal for hypertrophy in cardiac muscle. Proc Natl Acad Sci. 1998;95:5590–4.
Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6:182–7.
Zhang X, Zhang J, Kuang P, Lang S, Wu W, et al. The effect of lumbrokinase on P-selectin and E-selectin in cerebral ischemia model of rat. J Tradit Chin Med. 2003;23:141–6.
Kidwell CS, Saver JL, Mattiello J, Starkman S, Vinuela F, Duckwiler G, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol. 2000;47(4):462–9.
Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(8):e109–37.
Schellinger PD, Jansen O, Fiebach JB, Heiland S, Steiner T, Schwab S, et al. Monitoring intravenous recombinant tissue plasminogen activator thrombolysis for acute ischemic stroke with diffusion and perfusion MRI. Stroke. 2000;31(6):1318–28.
Kuntz M, Mysiorek C, Pétrault O, Petrault M, Uzbekov R, Bordet R, et al. Stroke-induced brain parenchymal injury drives blood–brain barrier early leakage kinetics: a combined in vivo/in vitro study. J Cereb Blood Flow Metab. 2014;34:95–107.
O’Collins VE, Macleod MR, Donnan GA, Horky LL, Van Der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–77.
Rosenberg N, Chen M, Prabhakaran S. New devices for treating acute ischemic stroke. Recent Pat CNS Drug Discov. 2005;5:118–34.
Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci. 2012;1268:21–5.
Franko J, Pomfy M, Prosbova T, Kimakova T. Temporal kinetics of calcium in neocortical neuronal nuclei after global cerebral ischemia and reperfusion. An ultrastructural study. Sb Lek. 2001;102(2):149–52.
Summerfield SG, Read K, Begley DJ, Obradovic T, Hidalgo IJ, Coggon S. Central nervous system drug disposition: the relationship between in situ brain permeability and brain free fraction. J Pharmacol Exp Ther. 2007;322:205–13.
Avdeef A. Physicochemical profiling (solubility, permeability and charge state). Curr Top Med Chem. 2001;1:277–351.
Laquintana V. New strategies to deliver anticancer drugs to brain tumors. Expert Opin Drug Deliv. 2009;6(10):1017–32.
Pardridge W. Vector mediated drug delivery to the brain. Adv Drug Deliv Rev. 1999;36:299–321.
Benedict CR, Refino CJ, Keyt BA, Pakala R, Paoni NF, Thomas GR, et al. New variant of human tissue plasminogen activator (TPA) with enhanced efficacy and lower incidence of bleeding compared with recombinant human TPA. Circulation. 1995;92:3032–40.
Armstead WM, Riley J, Yarovoi S, Cines DB, Smith DH, Higazi AA. TPA-S481A prevents neurotoxicity of endogenous tPA in traumatic brain injury. J Neurotrauma. 2012;29(9):1794–802.
Graham EM, Sheldon RA, Flock DL, Ferriero DM, Martin LJ, O’Riordan DP, et al. Neonatal mice lacking functional Fas death receptors are resistant to hypoxic-ischemic brain injury. Neurobiol Dis. 2004;17(1):89–98.
Maier W, Bednorz M, Meister S, Roebroek A, Weggen S, Schmitt U, et al. LRP1 is critical for the surface distribution and internalization of the NR2B NMDA receptor subtype. Mol Neurodegener. 2013;8:25.
Kanwar JR, Sriramoju B, Kanwar RK. Neurological disorders and therapeutics targeted to surmount the blood–brain barrier. Int J Nanomedicine. 2012;7:3259–78.
Pardridge WM. Biopharmaceutical drug targeting to the brain. J Drug Target. 2010;18(3):157–67.
Ren X, Cui G, Zhao M, Wang C, Peng S. Coordination of thrombolytic pro-ala-lys peptides with Cu (II): leading to nanoscale self-assembly, increase of thrombolytic activity and additional vasodilation. J Phys Chem B. 2008;112(27):8174–80.
Marsh JN, Hu G, Scott MJ, Zhang H, Goette MJ, Gaffney PJ, et al. A fibrin-specific thrombolytic nanomedicine approach to acute ischemic stroke. Nanomedicine (London). 2011;6(4):605–15.
Marsh JN, Partlow KC, Abendschein DR, Scott MJ, Lanza GM, Wickline SA. Molecular imaging with targeted perfluorocarbon nanoparticles: quantification of the concentration dependence of contrast enhancement for binding to sparse cellular epitopes. Ultrasound Med Biol. 2007;33(6):950–8.
Alexandrov AV, Demchuk AM, Burgin WS, Robinson DJ, Grotta JC, CLOTBUST Investigators. Ultrasound-enhanced thrombolysis for acute ischemic stroke: phase I. Findings of the CLOTBUST trial. J Neuroimaging. 2004;14(2):113–7.
Alexandrov AV. Ultrasound-enhanced thrombolysis for stroke: clinical significance. Eur J Ultrasound. 2002;16(1–2):131–40.
Alexandrov AV. Ultrasound enhanced thrombolysis for stroke. Int J Stroke. 2005;1(1):26–9.
Eibel B, Rodrigues CG, Giusti II, Nesralla IA, Prates PR, Sant Anna RT, et al. Gene therapy for ischemic heart disease: review of clinical trials. Rev Bras Cir Cardiovasc. 2011;26(4):635–46.
Masumu M, Hata R. Recent advances in adenovirus-mediated gene therapy for cerebral ischemia. Curr Gene Ther. 2003;3(1):43–8.
Yenari MA, Dumas TC, Sapolsky RM, Steinberg GK. Gene therapy for treatment of cerebral ischemia using defective herpes simplex viral vectors. Ann N Y Acad Sci. 2001;939:340–57.
Coles JP, Fryer TD, Smielewski P, Chatfield DA, Steiner LA, Johnston AJ, et al. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J Cereb Blood Flow Metab. 2004;24(2):202–11.
Soler EP, Ruiz VC. Epidemiology and risk factors of cerebral ischemia and ischemic heart diseases: similarities and differences. Curr Cardiol Rev. 2010;6(3):138–49.
European Stroke Organisation. ESO guidelines for the management of ischaemic stroke. Basel: European Stroke Organisation; 2008.
Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4(2):103–212.
Teeling JL, Perry VH. Systemic infection and inflammation in acute CNS injury and chronic neurodegeneration: underlying mechanisms. Neuroscience. 2009;158(3):1062–73.
Mattson MP, Duan W, Pedersen WA, Culmsee C. Neurodegenerative disorders and ischemic brain diseases. Apoptosis. 2001;6(1–2):69–81.
Goldstein LB. Modern medical management of acute ischemic stroke. Methodist Debakey Cardiovasc J. 2014;10(2):99–104.
Johnston DCC, Goldstein LB. Clinical carotid endarterectomy decision making: noninvasive vascular imaging versus angiography. Neurology. 2001;56(8):1009–15.
Albers GW, Caplan LR, Easton JD, Fayad PB, Mohr JP, Saver JL, et al. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347(21):1713–6.
Kidwell CS, Latour L, Saver JL, Alger JR, Starkman S, Duckwiler G, et al. Thrombolytic toxicity: blood brain barrier disruption in human ischemic stroke. Cerebrovasc Dis. 2008;25(4):338–43.
Kastrup A, Groschel K, Ringer TM, Redecker C, Cordesmeyer R, Witte OW, et al. Early disruption of the blood–brain barrier after thrombolytic therapy predicts hemorrhage in patients with acute stroke. Stroke. 2008;39(8):2385–7.
Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284(22):2901–6.
Gorelick PB. The burden and management of TIA and stroke in government-funded healthcare programs. Am J Manage Care. 2009;15(6 Suppl):S177–84.
Power A, Epstein D, Cohen D, Bathula R, Devine J, Kar A, et al. Renal impairment reduces the efficacy of thrombolytic therapy in acute ischemic stroke. Cerebrovasc Dis. 2013;35(1):45–52.
Masrur S, Abdullah AR, Smith EE, Hidalgo R, El-Ghandour A, Rordorf G, et al. Risk of thrombolytic therapy for acute ischemic stroke in patients with current malignancy. J Stroke Cerebrovasc Dis. 2011;20(2):124–30.
Ross OA, Worrall BB, Meschia JF. Advancing stroke therapeutics through genetic understanding. Curr Drug Targets. 2007;8(7):850–9.
Robinson T, Zaheer Z, Mistri AK. Thrombolysis in acute ischaemic stroke: an update. Ther Adv Chronic Dis. 2011;2(2):119–31.
Alyautdin R, Khalin I, Nafeeza MI, Haron MH, Kuznetsov D. Nanoscale drug delivery systems and the blood–brain barrier. Int J Nanomedicine. 2014;9:795–811.
Chen Y, Liu L. Modern methods for delivery of drugs across the blood–brain barrier. Adv Drug Deliv Rev. 2012;64(7):640–65.
Alavijeh MS, Chishty M, Qaiser MZ, Palmer AM. Drug metabolism and pharmacokinetics, the blood–brain barrier, and central nervous system drug discovery. NeuroRx. 2005;2(4):554–71.
Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the multi MERCI trial. Stroke. 2008;39:1205–12.
Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–8.
Pereira VM, Gralla J, Davalos A, Bonafé A, Castaño C, Chapot R, et al. Prospective, multicenter, single-arm study of mechanical thrombectomy using solitaire flow restoration in acute ischemic stroke. Stroke. 2013;44(10):2802–7.
Gralla J, Schroth G, Remonda L, Nedeltchev K, Slotboom J, Brekenfeld C. Mechanical thrombectomy for acute ischemic stroke, thrombus–device interaction, efficiency, and complications in vivo. Stroke. 2006;37:3019–24.
Mijajlovic MD, Pavlovic A, Covickovic-Sternic N. Is sonothrombolysis an effective stroke treatment? J Ultrasound Med. 2013;32:1117–23.
Rubiera M, Alexandrov AV. Sonothrombolysis in the management of acute ischemic stroke. Am J Cardiovasc Drugs. 2010;10(1):5–10.
Barlinn K, Alexandrov AV. Sonothrombolysis in ischemic stroke. Curr Treat Options Neurol. 2013;15(2):91–103.
Ahadi G, Welch CS, Grimm MJ, Fisher DJ, Zadicario E, Ernstrom K, et al. Transcranial sonothrombolysis using high-intensity focused ultrasound: impact of increasing output power on clot fragmentation. J Ther Ultrasound. 2013;1:22.
ACKNOWLEDGMENTS
The author thanks the Principal and Management, R.V.R. & J.C. College of Engineering (A), Guntur, Andhra Pradesh, India for their support to carry out the research work. Furthermore, my deep gratitude to the Department of Biotechnology, R.V.R. & J.C. College of Engineering (A) for providing facility utilized in the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pulicherla, K.K., Verma, M.K. Targeting Therapeutics Across the Blood Brain Barrier (BBB), Prerequisite Towards Thrombolytic Therapy for Cerebrovascular Disorders—an Overview and Advancements. AAPS PharmSciTech 16, 223–233 (2015). https://doi.org/10.1208/s12249-015-0287-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-015-0287-z