Abstract
This work aimed at improving the solubility of curcumin by the preparation of spray-dried ternary solid dispersions containing Gelucire®50/13-Aerosil® and quantifying the resulting in vivo oral bioavailability and anti-inflammatory activity. The solid dispersion containing 40% of curcumin was characterised by calorimetry, infrared spectroscopy and X-ray powder diffraction. The solubility and dissolution rate of curcumin in aqueous HCl or phosphate buffer improved up to 3600- and 7.3-fold, respectively. Accelerated stability test demonstrated that the solid dispersion was stable for 9 months. The pharmacokinetic study showed a 5.5-fold increase in curcumin in rat blood plasma when compared to unprocessed curcumin. The solid dispersion also provided enhanced anti-inflammatory activity in rat paw oedema. Finally, the solid dispersion proposed here is a promising way to enhance curcumin bioavailability at an industrial pharmaceutical perspective, since its preparation applies the spray drying, which is an easy to scale up technique. The findings herein stimulate further in vivo evaluations and clinical tests as a cancer and Alzheimer chemoprevention agent.
Similar content being viewed by others
INTRODUCTION
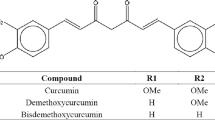
Curcumin, Fig. 1, is the main curcuminoid isolated from Curcuma longa L., (1) and has already been tested as an anti-inflammatory, hepatoprotective, antiviral, antibiotic, anticholesterolemic, antioxidant, neuroprotective, antitumoral (2–5) and as a chemopreventive for cancer and Alzheimer’s disease. Short-term clinical runs (6) demonstrated very low toxicity in a single daily doses of 12 and 8 g for 3 months. However, curcumin low solubility in aqueous solutions (7,8), instability in alkaline pH and short half-life lead to low oral bioavailability and therapeutic limitations (8–16). Curcumin solubility is a determining factor for its bioavailability when administered orally (17) and huge scientific work has been devoted to overcome this limitation (8,18–22).
Solid dispersion, SD, is an established concept for drug solubility and bioavailability enhancement (12,18,23–27) but strongly dependent on the choice of an appropriate carrier and preparation technique, which should lead to the formation of a stable dispersion (28) and allow the scale up in an adequate pharmaceutical perspective (29).
Among the studies on curcumin solid dispersions, curcumin-PVP resulted in complete drug dissolution within 30 min in water (12) and a 100-fold increase in drug solubility (30). Curcumin-Gelucire® 44/14 dispersions prepared by hot melt granulation (31) and spray drying (32) showed solubility increases of 4600- and 3200-fold, respectively. Despite their high solubility enhancement, the drug load was very low in both cases, limiting their use in solid oral dosage forms (33). Cellulose acetate-mannitol dispersion (22) was capable of increasing curcumin solubility 4-fold but in an aqueous media containing 15% ethanol. Cyclodextrins inclusion complexes were also evaluated and resulted in about 202-fold curcumin solubility improvement (10) and faster dissolution rates. However, none of these papers addressed the stability of curcumin solid dispersions, which is an important factor for pharmaceutical purposes (22,25,28) since recrystallization may decrease drug solubility with time (27). Moreover, SD physical-chemical characterisation and stability are rarely addressed along with in vitro and in vivo tests in solid dispersion studies (34).
Based on the results of previous works (31,32), Gelucire® solid dispersion obtained by spray drying showed excellent curcumin solubility enhancement, but there is lack of studies on the SD stability and bioavailability. The work reported here aimed to prepare highly loaded curcumin-Gelucire®50/13 SD by spray drying and characterise its solubility, dissolution rate, stability, pharmacokinetic and in vivo anti-inflammatory activity.
MATERIAL AND METHODS
Materials
Curcumin was purchased from Asian Herbex Ltd. (Hyderabad, India). Gelucire®50/13 (stearoyl macrogol-32 EP) was generously donated by Gattefosse Corp. (Saint-Priest, France), and the colloidal silicon dioxide Aerosil® was acquired from Evonik AG (Darmstadt, Germany). Analytical grade curcumin and the internal standard Sulindac were obtained from Sigma-Aldrich (St. Louis, MO, USA). Methanol was purchased from J.T. Baker (Phillipsburg, NJ, USA), and acetonitrile and acetic acid were obtained from Merck (Darmstadt, Germany). All solvents were HPLC grade.
Preparation of the Solid Dispersion
The preparation of solid dispersions followed the method optimised by Teixeira (31). Gelucire®50/13, GLC, was melted in a water bath, and a solution of curcumin, CUR, in ethanol:water (1:1) was added (GLC:CUR, 1:1). This mixture was homogenised with a high shear mixer at 18,000 rpm, and 20% (w/w) Aerosil was slowly added over approximately 1 min. Further homogenisation using a high shear mixer (14,000 rpm) was performed for 7 min. The suspension obtained by this procedure was dried in a lab-scale spray dryer model MSD 0.5 (Labmaq Ltda., Ribeirão Preto, Brazil) using the following set conditions: suspension feed rate of 5 mL/min, atomisation air pressure of 4 kgf/cm2, drying air rate of 1.5 m3/min, air outlet temperature of 40°C and a suspension solids content of 7.5% (w/w).
The physical mixture (PM) was prepared by simply mixing the components in a plastic bag using the same proportions of contends used in the SD formulation.
Physical-Chemical Characterisation and Stability of SD
The SD were characterised by particle size, water activity and curcumin solubility. Additionally, unformulated curcumin (UCUR), PM and SD physical-chemical properties were characterised by scanning electron microscopy (SEM), differential scanning calorimetry (DSC), thermogravimetry (TG), infrared spectroscopy (FTIR) and X-ray powder diffraction (XRPD). The stability was evaluated by observing the solubility of samples just after preparation (SD-0) after 3 (SD-3), 6 (SD-6) and 9 (SD-9) months of storage at room temperature (25°C), 70% RH, in triplicate and assessed by DSC, TG, XRPD and FTIR for 6 months (35).
Quantification of Curcumin
Curcumin was quantified by spectrophotometry using a standard curve prepared with seven different concentrations of analytical grade curcumin with 94% purity (Sigma-Aldrich, St. Louis, MO, USA). The maximum absorbance wavelength, 425 nm, was determined from the UV-Vis spectra, and it was in agreement with the literature (36). The readings were performed in a M330 Plus spectrophotometer (Camspec Ltd., Garforth, UK).
Particle Size, Flow, Morphology and Water Activity
The particle size of SD was determined in triplicate by sieving 50-g samples using 8 Tyler series sieves with aperture sizes from 250 to 1700 μm and a shaker (Bertel Ltda, Caieiras, Brazil). The mean diameter, D50, was calculated from the cumulative size distribution. Morphology was determined by SEM in a Jeol JSM 5200 scanning electron microscope (Jeol Ltd., Tokyo, Japan) for powder samples coated with a gold layer in a Baltec SCD 50 sputter coater (Furstentum Lichstenstein). Bulk and tapped densities were measured (USP XXX, 2007) and used to calculate the Carr Index and Hausner Factor. The powder angle of repose was also determined by the funnel method (37). Water activity was measured using a Testo 650 (Testo AG, Germany).
Thermal Analyses
DSC curves were obtained using a DSC 50 (Schimadzu Ltd., Kyoto, Japan). Five milligrammes of SD, PM, curcumin, Gelucire®50/13 and Aerosil® were weighed directly in aluminium pans using an Explorer analytical balance (Ohaus Co, USA). Pans were sealed, and the analyses were performed from 25 to 300°C in an inert atmosphere (N2 flow 50 mL/min) with a heating rate of 10°C/min. TG curves were obtained using 5-mg samples under N2 flux from 25 to 300°C using a TGA-50 (Schimadzu Ltd., Kyoto, Japan).
HSM was performed in a THMS600 microscope heating stage (Linkam Ltd., Surrey UK) with a Nikon microscope adapted with a Samsung DC camera and Axiovision software (Carl Zeiss AG, Jena, Germany). Temperature of the stage was increased from room to 90°C at 3°C/min rate with stops for 5 min at 30, 60 and 90°C for detailed analysis and image registration.
Fourier Transform Infrared Spectroscopy
FTIR was conducted by compacting the samples in KBr and analysing them in a Spectrum RXIFP-IR (Perkin Elmer, Waltham, USA). The spectra were recorded in the range of 4000 to 400 cm−1, and the characteristic peaks were identified and compared.
X-ray Powder Diffraction
XRPD was performed in a model D-5005 diffractometer (Siemens AG, Munich, Germany) fitted with a copper sealed source of 2.2 kW. The analyses were run at angles 2θ from 2 to 50° with an angle variation of 0.02°/s while applying 40 kV and 0.3 A.
Curcumin Dosage
The content was made by dissolving a quantity of SD in a mixture of water:ethanol 96% (1:1). The solution was filtered and the supernatant diluted in water for quantification by IR spectroscopy in the UV-visible spectrum. The content was given in milligrammes of curcumin per gram of dispersion. The recovery was calculated using the amount of curcumin theoretically expected as 100%.
Solubility
The curcumin solubility in water was determined in triplicate according to the method described by Damian et al. (38). Curcumin, the SD and the PM were all suspended in excess in Milli-Q® water (Millipore Inc., USA) in a 125-ml Erlenmeyer flask so that the media were saturated with curcumin. The quantities were calculated to a curcumin concentration of 25 mg/mL in water for all solubility experiments. The suspensions were kept under magnetic stirring in an AB/LM thermostatic air bath (Labmaq Ltd, Ribeirão Preto, Brazil) at 37 ± 0.5°C. After 48 h, aliquots were withdrawn and submitted to centrifugation at 3500 rpm for 10 min in a model 5.500D centrifuge (Cientec Ltd., Piracicaba, Brazil). The supernatants were filtered with 0.22-μm membranes (Millipore Inc., USA) and properly diluted, and the curcumin concentration was determined in an M330Plus UV-Vis spectrophotometer (Camspec Ltd., UK). The solubility of curcumin in the SD was observed for 9 months for stability evaluation.
Dissolution
The dissolution test was performed with curcumin, the PM and the SD-0. Hard gelatin capsules (size 1) were filled with the samples, standardised to a curcumin content of 20 mg per capsule and inserted in apparatus 1 (37). The dissolution test was performed in a model 299/6 dissolutor (NovaEtica Ltd., Brazil) under sink conditions. The apparatus was kept at a speed of 100 rpm and a temperature of 37.0 ± 0.5°C. The dissolution tests were performed in solutions of 0.1 M HCl and phosphate buffer solutions of pH 5.8 and pH 7.4. The tests were conducted in sextuplicate for each sample.
Pharmacokinetic Study in Rat
The experimental protocols for this study were approved by the Ethics Committee for Animal Use (CEUA/USP). Healthy male Wistar rats with an average body weight of 200 ± 20 g were obtained from the University of Sao Paulo breeding facilities. Rats were housed in polycarbonate cages with four animals in each cage under standard room temperature (22 ± 2°C) and humidity (55 ± 10%) and a 12-h light/dark cycle. Water and food were given ad libitum.
After the adaptation period, animals received a single administration of either curcumin or SD at 500 mg/kg in water suspension by oral gavage. At 15, 30, 60 and 120 min after oral gavage, whole blood was collected into heparinised tubes and centrifuged immediately at 3000g for 10 min. Plasma was then collected and stored at −20°C until analysis.
Sample Preparation and HPLC Analysis
A high performance liquid chromatography (HPLC) methodology was developed and validated for curcumin analysis in rat plasma. Briefly, 1 mL of plasma was pipetted into glass tubes and fortified with appropriate methanolic calibrators and an internal standard. Proteins were precipitated with 2 mL of acetonitrile and mixed at 2000 rpm for 1 min. The tubes were centrifuged at 2500g for 5 min, and supernatants were decanted into glass tubes and evaporated until dry. Samples were reconstituted with 100 μL of mobile phase, and 20 μL of the sample was injected into the HPLC. A linear curve generated from analysis of six different standard concentrations was constructed daily with r 2 ≥ 0.99.
Curcumin was quantified by an isocratic HPLC method using UV detection at a wavelength of 420 nm. The chromatographic analysis was performed in an RP-HPLC system consisting of a SHIMADZU LC-10AT VP chromatograph with a LiChrocart® C18 column (150 × 4,6 mm), 5-μm particle size (Merck, Damstadt, Germany), C-R8A integrator (Shimadzu, Japan) and Shimadzu SPD-10A ultraviolet detector. The mobile phase was methanol:deionised water:acetic acid (68:30.4:1.6 v/v) delivered at a flow rate of 1.0 mL/min.
Data Analysis
Compounds were identified based on acceptable retention time, peak shape and signal to noise ratio. Calibrator concentrations were required to be within ±15% of target concentration and ±20% for the limit of quantification.
The results presented are expressed as the mean ± standard deviation (n = 6). Data among different formulations were compared for statistical significance (p < 0.05) by one-way ANOVA, followed by Dunnett’s multiple comparison tests, and the SD was compared to UCUR using GraphPad Instat software version 5.03 (GraphPad Software, San Diego, CA).
Anti-Inflammatory Activity
The study of the anti-inflammatory activity of the SD-0 and curcumin in vivo was conducted using the rat paw oedema test at the Laboratory of Pharmacology of FCFRP. This experimental protocol was also submitted to and approved by the Ethics Committee, CEUA/USP (file number 09.1.1171.53.4).
Rats were housed in polycarbonate cages with up to 5 animals in each cage under standard room temperature (24 ± 2°C) and humidity (55 ± 10%) and a 12-h light/dark cycle. Water and food were given ad libitum. Seventy-two healthy male Wistar rats weighing 180 to 200 g were supplied by the Biotery 1 of FCFRP/USP and split into 8 groups composed of 9 animals each. Paw oedema was induced by hind left foot pad injection of 50 μL (10 mg/paw) of carrageenan (λ-carrageenan, type IV, Sigma-Aldrich, St. Louis, MO, USA) in 1% saline solution, and the contralateral paw (right) was injected with the same volume of saline solution. Drugs were administered after 2 h (120 min) by gavage according to the following groups: group 1, control (saline); group 2, indomethacin (5 mg/kg); group 3, curcumin (10 mg/kg); group 4, curcumin (30 mg/kg); group 5, curcumin (100 mg/kg); group 6, SD-0 (10 mg/kg); group 7, SD-0 (30 mg/kg) and group 8, SD-0 (100 mg/kg).
The volumes of both paws of each animal were measured using a water plethysmometer (Ugo Basile, Italy) before (1 and 2 h) and after the injection of carrageenan and 30, 60, 120, and 180 min after the administration of curcumin, SD and indomethacin. The formation of oedema was expressed by the differences between the volumes (mL) of the left and right paws. The data were statistically analysed by the Tukey’s t test. After the experiment, the animals were euthanised in a CO2 chamber.
RESULTS
The SD were previously characterised for their pharmaceutics properties. The size distribution revealed the prevalence of small particles, as 69% of them were stranded in a 600-μm sieve, resulting in a mean diameter, D50, of 550 μm.
The photomicrographs of curcumin and the SD by SEM are illustrated in Fig. 2. The crystal rhomboid needle-like structure of pure curcumin is shown in Fig. 2a. In Fig. 2b, c, one can observe that the microparticulated dispersions are present in a round but not perfectly spherical shape. Additionally, the microparticles seem to be porous or composed of agglomerates, Fig. 2c.
The measured angle of repose for SD microparticles was 25°, the Carr index was 9.53%, and the Hausner factor was 1.10. Furthermore, the coefficient of water activity was 0.315.
The content of curcumin in the SD quantified by UV-Vis spectrometry was 338.4 mg of the drug per gram of the dispersion. This figure was used to calculate the actual content of curcumin in the hard gelatin capsules used for the dissolution study.
Physical-Chemical Characterisation and Stability of SD
The thermal analysis results can be observed in Fig. 3, which shows DSC results only, and Table I. In Fig. 3, the DSC curves of Gelucire®50/13, Aerosil®, curcumin, the PM and the SD are illustrated. The melting points of curcumin and Gelucire®50/13 are 172 and 46.4°C, respectively. As shown in Fig. 3, there are no changes in the main events, temperatures or intensities of the peaks for SD-0, SD-3 and SD-6. This is confirmed by the data in Table I, which shows the physical stability in formulation.
TGA was also performed for all samples. For the sake of conciseness, the curves were omitted, but the main results are summarised in Table I. As shown in this table, the thermal decomposition of both the SD and the PM occurred in a single event, and the same was observed for curcumin. Table I shows the percent weight loss for curcumin, the SD and the PM within the temperature range of 220 to 299°C. It is possible to verify that the energy variation observed for the SD and the PM in the DSC curves (at 126.7 and 135.6°C, respectively) in Fig. 3 are not accompanied by the loss of mass in TG analysis according to Table I. As showns in Table I, there are no differences for SD-0, SD-3 and SD-6; however, to verify more precisely where there were physical or chemical interactions, FTIR analysis was performed.
The spectra of samples of curcumin, Gelucire®50/13, Aerosil®, the SD and the PM are shown in Fig. 4. Curcumin is a bis-α-β-unsaturated β-diketone with two methoxyphenyl rings in its structure (Fig. 1). In the FTIR spectrum of curcumin, the peak at 1628 cm−1 corresponds to the carbonyl group, whereas the bands in 1601, 1585, 1509 and 1456 cm−1 can be attributed to the vibration stretching of the double bonds (C=C) from the benzene ring. Additionally, the peak at 3517 cm−1 is relative to the hydroxyl group bonded to the benzene ring (Fig. 4). In the PM and SD-0 spectra, the bands related to the stretching of (C=C) of the benzene ring not only appear expanded but they also seem to have moved to 1512, 1511 and 1510 cm−1, respectively. These bands correspond to the peak at 1509 cm−1 in the curcumin spectrum that has shifted to a higher frequency. Benzene rings with electron donor character usually show a broad band at 1510 cm−1.
As can be observed, Fig. 4, there was no change in the spectra for SD-0, SD-3 and SD-6.
The XRPD can be observed in Fig. 5. The curcumin and Gelucire®50/13 peaks can also be observed in the PM and SD-0 with a decrease in their intensities. The diffraction patterns in Fig. 5 show that the peaks were not altered during the storage, as the spectra were equal for SD-0, SD-3 and SD-6.
Solubility and Dissolution
Figure 6 presents the solubility of curcumin, the PM and the SD. The solubility of curcumin was determined to be 0.75 μg/mL, whereas the PM resulted in a curcumin solubility of 369 μg/mL, indicating that the presence of the carrier in the solution enhanced solubility. The solubility determined for curcumin from the SD-0, however, is 2700 μg/mL, indicating an increase of 3600-fold compared to UCUR and 7.3-fold compared to the PM. Additionally, the data in Fig. 6 confirm that the SD was stable during 9 months of storage under controlled conditions. The decrease in SD solubility was 4.0, 5.2 and 5.4% after 3 (SD-3), 6 (SD-6) and 9 (SD-9) months, respectively. Although it seems to decrease with time, the stability test for solubility was run in triplicate samples, and statistical analysis (t test) demonstrated that the differences between SD-0 and other samples were not significant at a 5% level (p < 0.05).
The in vitro dissolution profile study provides information on the speed and extension of the active release. The curves in Fig. 7 show that in the initial 10 min of the test, the percentage of curcumin released was approximately 90% at pH 1.2 and 5.8 and 73% at pH 7.4 for the SD, whereas the release of PM and UCUR was so low that it was not possible to quantify. The maximum amount of curcumin released from SD was reached in 10 min and corresponded to 90% at pH 1.2 and 5.8 and 80% at pH 7.4. The maximum amount of curcumin release from PM was 37% at pH 1.2, 42% at pH 5.8 and 36% at pH 7.4, showing that the release from SD were about 2-fold those of PM in different dissolution media. The maximum amount of curcumin released from UCUR was approximately 20%.
IN VIVO STUDIES
Pharmacokinetics in Rat
The plasma concentrations of curcumin in animals that received either curcumin or the SD were determined. Rats received curcumin or SD at 500 mg/kg by oral gavage, and the plasma was obtained at time points up to 2 h after administration. The SD significantly increased curcumin levels in plasma compared to concentrations measured in animals that received UCUR (Fig. 8). Peak plasma levels of curcumin were approximately 5-fold higher for the SD than for the unformulated curcumin. The highest plasma curcumin concentration following administration of UCUR was 3.2 ± 1.4 ng/mL, whereas a peak plasma concentration of 17.6 ± 9.4 ng/mL after administration of the SD formulation of curcumin was observed.
Plasma concentrations of curcumin in rats that received a single dose of unformulated curcumin (dotted line) or curcumin in solid dispersion (solid line) at 500 mg/kg by oral gavage. * indicates that at that time point, values in solid dispersion were significantly different from those of unformulated curcumin. This was determined by a one-way ANOVA, followed by Dunnett’s test (p < 0.05)
Anti-Inflammatory Activity
The formation of oedema was expressed by the difference between the volumes of right and left paws in millilitres. The larger the volume difference, the greater the oedema, as only the right paw received the inflammatory stimulus, i.e., the carrageenan injection.
Figure 9a shows the formation of paw oedema in groups that received 10, 30 and 100 mg/kg of curcumin, indomethacin (5 mg/kg) and the control group (saline solution). As observed at the maximum peak of inflammation (3 h after administration of carrageenan), there was a significant difference between the groups that received curcumin and the control group, confirming the anti-inflammatory activity of curcumin. Indomethacin is an anti-inflammatory that is widely prescribed, and it had the highest decrease in ΔV at 5 mg/kg.
Rat paw oedema. a Rat paw oedema for different doses of unformulated curcumin and the control drug indomethacin. * indicates significance at 5% for unformulated curcumin and control (indomethacin). b Rat paw oedema for groups administered unformulated curcumin (100 mg/kg), dispersion DS17 (100 mg/kg) and indomethacin (10 mg/kg). * indicates significance at 0.5% for DS/indomethacin; **, 0.1% for DS/curcumin; ***, 1% for control/indomethacin. c Rat paw oedema for different doses of dispersion DS17. * and ** indicate significance at 0.5 and 0.1%, respectively, for DS 17 and control (indomethacin)
Figure 9b presents the paw oedema for the control group compared to groups that received 100 mg/kg curcumin, 100 mg/kg SD and 5 mg/kg indomethacin. As shown 1, 2 and 3 h after the administration of SD, there was a significant (p < 0.01) difference between this group and the one that received unformulated curcumin, as the SD exhibited a considerably higher level of anti-inflammatory activity at the doses studied. It must be remembered that the curcumin content in the SD was determined to be 338.4 mg/g (33.8% w/w), indicating that the higher bioavailability is reflected in the more effective anti-inflammatory activity. The SD was even more effective than the indomethacin in decreasing the formation of oedema under the conditions studied (p < 0.05). Additionally, UCUR at 100 mg/kg had anti-inflammatory action similar to that of indomethacin at 5 mg/kg.
Figure 9c shows the formation of paw oedema in the groups receiving different concentrations of the SD (SD 10, 30 and 100 mg/kg), indomethacin (5 mg/kg) and the control group. The anti-inflammatory activity, i.e., DV decrease, appears to be dose-dependent, as DV decreased as the SD concentration increased from 10 to 100 mg/kg. Most importantly, 100 mg/kg SD was more effective at reducing oedema than 5 mg/kg indomethacin.
DISCUSSION
With increase in life expectancy, majority of people in the older age dwell with multiple disorders, thus, creating a need to look upon molecules with multiple targets. Curcumin is an antioxidant compound and targets multiple chemotherapeutic and inflammatory pathways and has demonstrated safety and tolerability in humans; however, the clinical literature lacks conclusive evidence supporting its use as a therapeutic agent due to its low bioavailability (39).
It is well known that many drugs show bioavailability problems due to their low water solubility, slow dissolution rate and instability in the gastrointestinal tract. Poor oral absorption due to its extremely low aqueous solubility or extensive presystemic metabolism may be responsible for the unfavourable pharmacokinetics of this molecule (40).
Considering the potential of solid dispersions as oral drug delivery systems, the present investigation involved development and characterisation of SD with a view to improve curcumin oral bioavailability. The SD was prepared with Gelucire®50/13 SD and Aerosil® by spray drying.
The SD were previously characterised for their pharmaceutics properties. The size distribution revealed the prevalence of micrometric particle sizes. Size is of paramount importance because it can influence the dissolution of the drug and the flow properties which are important in pharmaceutical manipulation. The microparticulated solid dispersion resulted in a mean size of 550 μm and repose angle of 25° which demonstrates suitability for further industrial application. Furthermore, SD water activity can be considered low and adequate for long microbiological stability.
Additionally, as a consequence of both spray drying and solution properties (41), the microparticles morphology showed to be porous or composed of agglomerates, as seen in SEM microphotographs. The primary or smaller particles varied both in size and shape due to the ternary composition of the SD, while the agglomerate structures are composed of individual sub-micron particles of the SD mixture. Furthermore, the porous and irregular structure of the microparticles should promote rapid dissolving in water. Curcumin crystals have been demonstrated to have a flat-needle shape with well-developed edges (12), but these structures cannot be observed in solid dispersions SEM, which indicates that the drug is intimately dispersed within the Gelucire®50/13 matrix.
The DSC curves provide information on thermal events characterised by a change in enthalpy in a range of temperatures, such as the melting, crystallisation and they can indicate physical or chemical interactions between components of the formulation. The melting points of curcumin and Gelucire®50/13 are 172 and 46.4°C, respectively. The melting point of curcumin depends on its relative crystallinity and has been reported to be 180°C according to Paradkar et al. (12) and 176.8°C according to Xie et al. (20). Table I shows the thermoanalytical data extracted from the DSC curves. The peaks corresponding to curcumin melting in the SD and PM appeared at lower temperatures. These data suggest that curcumin is partially dissolved in the SD at a molecular level, which may be caused by previous melting of Gelucire®50/13 at 46.4°C and consequent curcumin solubilisation in the melted carrier. Both curcumin and Gelucire®50/13 have non-polar characters which favours their interaction through intermolecular van der Waals forces. Additional energy is supplied to the process by heating, favouring these interactions between the drug and carrier and causing the solubilisation of the curcumin in the melted Gelucire®50/13. This phenomenon could be confirmed by hot stage microscopy, HSM, but the data is not shown here. The solubilisation of drug in the carrier explains the decrease in curcumin melting temperature observed by DSC, as melting of mixtures usually occur at temperatures lower than the respective melting points of pure components. In addition to the interaction of the components in SD preparation, thermal analysis is also suitable for stability evaluation because any physical changes in the SD can be detected by these methods. The DSC for SD obtained along 9 months showed no important changes, demonstrating the physical stability of the dispersion.
The confirmation of the physical or chemical nature of a thermal event may be observed through thermogravimetric analysis. TGA curves confirmed the main events corresponding to melting of the components, but most importantly they showed that SD mass loss begins above 220°C, demonstrating that the SD is thermally stable up to this temperature.
FTIR is a very sensitive methodology and most chemical changes can be detected by this method. The spectra obtained by FTIR for SD and PM suggest hydrogen bond interactions between the Gelucire®50/13. The FTIR spectra obtained along 90 days confirm the indications from the DSC and TGA analyses, suggesting good physical-chemical stability of the microparticles during the evaluation period.
Another important characterisation of the SD is the crystallinity of the samples based on XRPD. The main peaks for curcumin agree with the curcumin diffraction pattern presented by Paradkar et al. (12) and Xie et al. (20). The curcumin and Gelucire®50/13 peaks can also be observed in the PM and SD-0 with a decrease in their intensities. The decrease in peaks intensity can be attributed to both the decrease in crystallinity and the dilution of components in the PM and SD-0. The XRPD was also applied to evaluate the SD stability after 3 and 6 months and showed that the peaks were not altered during the storage.
The curcumin solubility in PM was increased 492-fold and this effect was expected because Gelucire®50/13 is a non-ionic, water dispersible and self-emulsifying surfactant capable of forming aqueous microemulsions that have been proven to improve the solubility of drugs. However, SD-0 resulted in a curcumin solubility increase of 3600-fold or 7.3-fold when compared to PM. A literature review showed curcumin solubility increases of 4-fold (22), 100-fold (12), 1000-fold (42), 4600-fold (31) and 3200-fold (32) when applying the solid dispersion concept. As a comparison, solubility increases of curcumin in cyclodextrin molecular inclusions were reported to be 31-fold (43), 190- to 200-fold (12) and 203-fold (39). Despite the best results in solubility improvement obtained by now were from ternary solid dispersions prepared by hot melt granulation (31) and spray drying (32), these forms had a very low curcumin load which difficult future applications in solid oral dosage forms (33). The remarkable solubility enhancement for SD-0, associated with the findings of DSC, HSC and FTIR suggests that the process of SD preparation was able to create a molecular dispersion of the drug within the carrier matrix, which was potentiated by hydrogen bonding. Additionally, the SD was stable during 6 months of storage under controlled conditions, as revealed by DSC, FTIR and XRPD and during 9 months as revealed by solubility tests. Instability is one of the limiting factors in the application of the SD concept for the increase of drug solubility (44), and the previous studies on curcumin solid dispersions (12,31,32,42) have not addressed this issue, but the form proposed here seem to be stable enough for pharmaceutical industrial perspective. The stability of the dispersion obtained by spray drying may also be due to the presence of hydrogen bonds between the curcumin and Gelucire®50/13 and their adsorption onto the surface of the Aerosil (45) .
The in vitro dissolution test provided information on the speed and extension of the active release. Therefore, the SD was able to improve exceptionally the dissolution rate of curcumin and the total amount released. Furthermore, after the curcumin release from SD reached its maximum, a plateau was observed, showing no decrease in solubilised curcumin until the test ended at 120 min. The rapid decrease in the concentration of curcumin in the dissolution media at pH 7.4 was observed by other authors (32) and could be caused by curcumin degradation at alkaline pH (46).
Rodents exhibit a poor oral systemic bioavailability of curcumin that is similar to what is observed in humans (13). Pharmacokinetic studies in rats describe the drastic reduction in plasma curcumin concentrations over a period of up to 2 h, and these studies have determined that the maximal plasma concentration of curcumin is achieved approximately 30 min after oral administration (47,48). A study in rats reported a plasma level of 2.4 ng/mL 30 min after administration of 340 mg/kg of curcumin by oral gavage (43). In our study, oral administration of 500 mg/kg of UCUR reached a maximal plasma concentration of 3.2 ng/mL in 30 min. Curcumin from SD, however, reached a maximal plasma concentration of 17.6 ng/mL in 60 min after oral gavage, which is approximately 5-fold higher than unformulated curcumin. A similar result in bioavailability increase, about 5-fold, was reported for curcumin-Solutol® HS solid dispersions (42); however, a low drug load (max 10%) was obtained by this form.
The UCUR showed anti-inflammatory activity; however, SD exhibited a considerably higher level of anti-inflammatory activity at the doses studied. This increase in anti-inflammatory activity can be explained by the greater solubility from SD as compared to UCUR, allowing greater absorption and bioavailability. Recently, the increase of solubility has been shown to improve the oral bioavailability of liposoluble drugs. The importance of water solubility and dissolution of liposoluble drugs piroxicam and ritonavir in improving their in vivo absorption has also been demonstrated (43,49). In addition, Gelucire®50/13 fatty acids or fatty acid esters could also increase the intestinal absorption of curcumin due to a possible increase in intestinal membrane permeability (30).
Finally, curcumin SD developed here is certainly advantageous over unformulated curcumin in pharmacotherapeutic interventions that target the gastrointestinal tract and maybe other sites, as there is consensus that the improved bioavailability may increase the potential medical applications of curcumin.
CONCLUSIONS
In this study, a ternary solid dispersion of Gelucire®50/13-Aerosil containing curcumin in a high load (40%) was developed and showed adequate pharmaceutics properties and a remarkable 3600-fold enhancement in curcumin solubility in water. The solid dispersion proposed was stable for 9 months in accelerated stability test as indicated by calorimetry, infrared spectroscopy, X-ray diffraction and curcumin solubility. The solubility, dissolution rates and drug load obtained in this work for curcumin solid dispersion were superior to that obtained in previous works. Furthermore, the bioavailability and anti-inflammatory activity of curcumin were highly improved by solid dispersion as a consequence of an increased gastrointestinal absorption.
Finally, the solid dispersion proposed here is a promising way to enhance curcumin bioavailability at an industrial pharmaceutical perspective, since its preparation applies the spray drying, which is an easy to scale up technique. The findings herein should stimulate further in vivo evaluations and clinical tests as a cancer and Alzheimer chemoprevention agent.
REFERENCES
Joshi P, Jain S, Sharma V. Turmeric (Curcuma longa) a natural source of edible yellow colour. Int J Food Sci Technol. 2009;44(12):2402–6. doi:10.1111/j.1365-2621.2009.01914.x.
Tilak JC, Banerjee M, Mohan H, Devasagayam TP. Antioxidant availability of turmeric in relation to its medicinal and culinary uses. Phytother Res. 2004;18(10):798–804. doi:10.1002/ptr.1553.
Jayaprakasha GK, Rao JM, Sakariah KK. Chemistry and biological activities of C. longa. Trends Food Sci Technol. 2005;16(12):533–48. doi:10.1016/j.tifs.2005.08.006.
Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78(18):2081–7. doi:10.1016/j.lfs.2005.12.007.
Chen F, Wang H, Xiang X, Yuan J, Chu W, Xue X, et al. Curcumin increased the differentiation rate of neurons in neural stem cells via wnt signaling in vitro study. J Surg Res. 2014;192(2):298–304. doi:10.1016/j.jss.2014.06.026.
Hamaguchi T, Ono K, Yamada M. Review: curcumin and Alzheimer’s disease. CNS Neurosci Ther. 2010;16(5):285–97. doi:10.1111/j.1755-5949.2010.00147.x.
Pfeiffer E, Höhle S, Solyom AM, Metzler M. Studies on the stability of turmeric constituents. J Food Eng. 2002;56(2-3):257–9.
Yadav D, Kumar N. Nanonization of curcumin by antisolvent precipitation: process development, characterization, freeze drying and stability performance. Int J Pharm. 2014;477(1-2):564–77. doi:10.1016/j.ijpharm.2014.10.070.
Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol. 2007;595:453–70.
Rachmawati H, Edityaningrum CA, Mauludin R. Molecular inclusion complex of curcumin-beta-cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPSPharmSciTech. 2013;14(4):1303–12. doi:10.1208/s12249-013-0023-5.
Ko WC, Chang CK, Wang HJ, Wang SJ, Hsieh CW. Process optimization of microencapsulation of curcumin in gamma-polyglutamic acid using response surface methodology. Food Chem. 2015;172:497–503. doi:10.1016/j.foodchem.2014.09.100.
Paradkar A, Ambike AA, Jadhav BK, Mahadik KR. Characterization of curcumin-PVP solid dispersion obtained by spray drying. Int J Pharm. 2004;271(1-2):281–6. doi:10.1016/j.ijpharm.2003.11.014.
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–900.
Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61(3):1058–64.
Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41(13):1955–68. doi:10.1016/j.ejca.2005.05.009.
Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, Bailey JM, et al. Dose escalation of a curcuminoid formulation. BMC Complem Altern M. 2006;6:10. doi:10.1186/1472-6882-6-10.
Dannenfelser RM, He H, Joshi Y, Bateman S, Serajuddin AT. Development of clinical dosage forms for a poorly water soluble drug I: application of polyethylene glycol-polysorbate 80 solid dispersion carrier system. J Pharm Sci. 2004;93(5):1165–75. doi:10.1002/jps.20044.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50(1):47–60. doi:10.1016/S0939-6411(00)00076-X.
Wang Y, Lu ZX, Lv FX, Bie XM. Study on microencapsulation of curcumin pigments by spray drying. Eur Food Res Technol. 2009;229(3):391–6. doi:10.1007/s00217-009-1064-6.
Xie X, Tao Q, Zou Y, Zhang F, Guo M, Wang Y, et al. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem. 2011;59(17):9280–9. doi:10.1021/jf202135j.
Ganta S, Talekar M, Singh A, Coleman TP, Amiji MM. Nanoemulsions in translational research—opportunities and challenges in targeted cancer therapy. AAPSPharmSciTech. 2014;15(3):694–708. doi:10.1208/s12249-014-0088-9.
Wan SX, Sun YQ, Qi XX, Tan FP. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPSPharmSciTech. 2012;13(1):159–66. doi:10.1208/s12249-011-9732-9.
Choonara BF, Choonara YE, Kumar P, du Toit LC, Tomar LK, Tyagi C, et al. A menthol-based solid dispersion technique for enhanced solubility and dissolution of sulfamethoxazole from an oral tablet matrix. AAPSPharmSciTech. 2014. doi:10.1208/s12249-014-0271-z.
Modi A, Tayade P. Enhancement of dissolution profile by solid dispersion (kneading) technique. AAPSPharmSciTech. 2006;7(3). doi: 10.1208/pt070368.
Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12(23-24):1068–75. doi:10.1016/j.drudis.2007.09.005.
Rajarajan SB, Baby B, Ramesh K, Singh D. Preparation and evaluation of ternary mixing itraconazole solid dispersion by spray drying method. Int J Pharm Sci Res. 2009;1:22–5.
Sethia S, Squillante E. Solid dispersions: revival with greater possibilities and applications in oral drug delivery. Crit Rev Ther Drug Carrier Syst. 2003;20(2-3):215–47. doi:10.1615/CritRevTherDrugCarrierSyst.v20.i23.40.
Schachter DM, Xiong J, Tirol GC. Solid state NMR perspective of drug-polymer solid solutions: a model system based on poly(ethylene oxide). Int J Pharm. 2004;281(1-2):89–101. doi:10.1016/j.ijpharm.2004.05.024.
Chow K, Tong HH, Lum S, Chow AH. Engineering of pharmaceutical materials: an industrial perspective. J Pharm Sci. 2008;97(8):2855–77. doi:10.1002/jps.21212.
Martins RM, Pereira SV, Siqueira S, Salomao WF, Freitas LAP. Curcuminoid content and antioxidant activity in spray dried microparticles containing turmeric extract. Food Res Int. 2013;50(2):657–63. doi:10.1016/j.foodres.2011.06.030.
Teixeira CCC. Desenvolvimento tecnológico de fitoterápico a partir de rizomas de Curcuma longa L. e avaliação das atividades antioxidante, anti-inflamatória e antitumoral. Ribeirão Preto: Universidade de São Paulo; 2009.
Araujo RR, Teixeira CCC, Freitas LAP. The preparation of ternary solid dispersions of an herbal drug via spray drying of liquid feed. Dry Technol. 2010;28(3):412–21. doi:10.1080/07373931003648540.
Okonogi S, Puttipipatkhachorn S. Dissolution improvement of high drug-loaded solid dispersion. AAPSPharmSciTech. 2006;7(2):E148–53. doi:10.1208/pt070252.
Yuksel N, Karatas A, Ozkan Y, Savaser A, Ozkan SA, Baykara T. Enhanced bioavailability of piroxicam using Gelucire 44/14 and labrasol: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2003;56(3):453–9. doi:10.1016/S0939-6411(03)00142-5.
Harmonisation. ICH. Guidance for industry Q1A(R2) stability testing of new drug substances and products. In: Dept. of Health and Human Services, editor. Rockville, MD : U.S. : Food and Drug Administration, Center for Drug Evaluation and Research; 2003.
Constant P, Stringheta P, Sandi D. CORANTES ALIMENTÍCIOS. Boletim do Centro de Pesquisa de Processamento de Alimentos [Internet]. 2005 17 Jan. 2012; 20(2):203-20.
Convention USP. USP XXX United States Pharmacopeia. USA: Rockville; 2007.
Damian F, Blaton N, Naesens L, Balzarini J, Kinget R, Augustijns P, et al. Physicochemical characterization of solid dispersions of the antiviral agent UC-781 with polyethylene glycol 6000 and Gelucire 44/14. Eur J Pharm Sci. 2000;10(4):311–22. doi:10.1016/S0928-0987(00)00084-1.
Kakkar V, Kaur IP. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem Toxicol. 2011;49(11):2906–13. doi:10.1016/j.fct.2011.08.006.
Yadav VR, Suresh S, Devi K, Yadav S. Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPSPharmSciTech. 2009;10(3):752–62. doi:10.1208/s12249-009-9264-8.
Walton DE. The morphology of spray dried particles: a qualitative view. Dry Technol. 2000;18(9):1943–86. doi:10.1080/07373930008917822.
Seo SW, Han HK, Chun MK, Choi HK. Preparation and pharmacokinetic evaluation of curcumin solid dispersion using Solutol (R) HS15 as a carrier. Int J Pharm. 2012;424(1-2):18–25. doi:10.1016/j.ijpharm.2011.12.051.
Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60(2):171–7. doi:10.1007/s00280-006-0355-x.
Gupta MK, Bogner RH, Goldman D, Tseng YC. Mechanism for further enhancement in drug dissolution from solid-dispersion granules upon storage. Pharm Dev Technol. 2002;7(1):103–12. doi:10.1081/PDT-120002236.
Chauhan B, Shimpi S, Paradkar A. Preparation and evaluation of glibenclamide-polyglycolized glycerides solid dispersions with silicon dioxide by spray drying technique. Eur J Pharm Sci. 2005;26(2):219–30. doi:10.1016/j.ejps.2005.06.005.
Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharmaceut Biomed. 1997;15(12):1867–76. doi:10.1016/S0731-7085(96)02024-9.
Zhongfa L, Chiu M, Wang J, Chen W, Yen W, Fan-Havard P, et al. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother Pharmacol. 2011. doi:10.1007/s00280-011-1749-y.
Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B. 2007;853(1-2):183–9. doi:10.1016/j.jchromb.2007.03.010.
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–18. doi:10.1021/mp700113r.
ACKNOWLEDGMENTS
Financial support from Fapesp (2011/20872-7) and CNPq (PQ-2) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teixeira, C.C.C., Mendonça, L.M., Bergamaschi, M.M. et al. Microparticles Containing Curcumin Solid Dispersion: Stability, Bioavailability and Anti-Inflammatory Activity. AAPS PharmSciTech 17, 252–261 (2016). https://doi.org/10.1208/s12249-015-0337-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-015-0337-6