Abstract
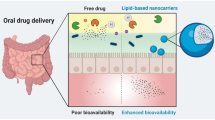
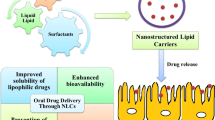
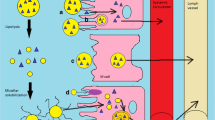
The therapeutic functionality of innumerable antiretroviral drugs is supposedly obscured owing to their low metabolic stability in the gastrointestinal tract and poor solubilization property leading to poor oral bioavailability. Dictated by such needs, lipid-based formulations could be tailored using nanotechnology which would be instrumental in ameliorating the attributes of such drugs. The stupendous advantages which lipid nanocarriers offer including improved drug stability and peroral bioavailability coupled with sustained drug release profile and feasibility to incorporate wide array of drugs makes it a potential candidate for pharmaceutical formulations. Furthermore, they also impart targeted drug delivery thereby widening their arena for use. Therefore, the review will encompass the details pertaining to numerous lipid nanocarriers such as nanoemulsion, solid lipid nanoparticle, nanostructured lipid carriers, and so on. These nanocarriers bear the prospective of improving the mucosal adhesion property of the drugs which ultimately upgrades its pharmacokinetic profile. The biodegradable and physiological nature of the lipid excipients used in the formulation is the key parameter and advocates for their safe use. Nevertheless, these lipid-based nanocarriers are amenable to alterations which could be rightly achieved by changing the excipients used or by modifying the process parameters. Thus, the review will systematically envisage the impending benefits and future perspectives of different lipid nanocarriers used in oral delivery of antiretroviral drugs.



Similar content being viewed by others
Abbreviations
- HIV:
-
Human immunodeficiency virus
- SLN:
-
Solid lipid nanoparticle
- NLC:
-
Nanostructured lipid carrier
- TG:
-
Triglyceride
- SNEDDS:
-
Self nano emulsifying drug delivery systems
- GIT:
-
Gastrointestinal tract
- GI:
-
Gastrointestinal
- HAART:
-
Highly active antiretroviral treatment
- BBB:
-
Blood–brain barrier
- BCB:
-
Blood cerebrospinal fluid barrier
References
Sant S, Tao SL, Fisher OZ, Xu Q, Peppas NA, Khademhosseini A. Microfabrication technologies for oral drug delivery. Adv Drug Deliv Rev. 2012;64:496–507.
Bak A, Leung D, Barrett SE, Forster S, Minnihan EC, Leithead AW, et al. Physicochemical and formulation developability assessment for therapeutic peptide delivery da primer. AAPS J. 2015;17:144–55.
Lin CH, Chen CH, Lin ZC, Fang JY. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J Food Drug Anal. 2017;25:219–34.
Bernkop-Schnurch A. Nanocarrier systems for oral drug delivery: do we really need them? Eur J Pharm Sci. 2013;49:272–7.
Kalepu S, Manthina M, Padavala V. Oral lipid-based drug delivery systems—an overview. Acta Pharm Sin B. 2013;3(6):361–72.
Chaudhary S, Garg T, Murthy RS, Rath G, Goyal AK. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target. 2014;22:871–82.
Khan AA, Mudassir J, Mohtar N, Darwis Y. Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomedicine. 2013;8:2733–44.
Gershkovich P, Hoffman A. Effect of a high-fat meal on absorption and disposition of lipophilic compounds: the importance of degree of association with triglyceride-rich lipoproteins. Eur J Pharm Sci. 2007;32:24–32.
Batista P, Castro PM, Madureira AR, Sarmento B, Pintado M. Recent insights in the use of nanocarriers for the oral delivery of bioactive proteins and peptides. Peptides. 2018;101:112–23. https://doi.org/10.1016/j.peptides.2018.01.002.
Sosnik A, Augustine R. Challenges in oral drug delivery of antiretrovirals and the innovative strategies to overcome them. Adv Drug Deliv Rev. 2013;103:105–20.
Pathak K, Raghuvanshi S. Oral bioavailability: issues and solutions via nanoformulations. Clin Pharmacokinet. 2015;54(4):325–57.
Muheem A, Anwar M, Shakeel F, Mallick N, Jahangir MA, Jain GK, et al. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm J. 2016;24:413–28.
Schenk M, Mueller C. The mucosal immune system at the gastrointestinal barrier. Best Pract Res Clin Gastroenterol. 2008;22:391–409.
Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64:557–70.
Alai MS, Lin WJ, Pingale SS. Application of polymeric nanoparticles and micelles in insulin oral delivery. J Food Drug Anal. 2015;23:351–8.
Karamanidou T, Bourganis V, Kammona O, Kiparissides C. Lipid-based nanocarriers for the oral administration of biopharmaceutics. Nanomedicine. 2016;11:3009–32. https://doi.org/10.2217/nnm-2016-0265.
Cao S, Woodrow KA. Nanotechnology approaches to eradicating HIV reservoirs. Eur J Pharm Biopharm. 2018. https://doi.org/10.1016/j.ejpb.2018.06.002.
Lenjisa JL, Woldu MA, Satessa GD. New hope for eradication of HIV from the body: the role of polymeric nanomedicines in HIV/AIDS pharmacotherapy. J Nanobiotechnol. 2014;12:9. https://doi.org/10.1186/1477-3155-12-9.
Crowe SM. Macrophages and residual HIV infection. Curr Opin HIV AIDS. 2006;1:129–33.
Amiji MM, Vyas TK, Shah LK. Role of nanotechnology in HIV/AIDS treatment: potential to overcome the viral reservoir challenge. Discov Med. 2006;6(34):157–62.
Mamo T, Moseman EA, Kolishetti N, Salvador-Morales C, Shi J, Kuritzkes DR, et al. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine. 2010;5:269–85. https://doi.org/10.2217/nnm.10.1.
Jiang Y, Cao S, Bright DK, Bever AM, Blakney AK, Suydam IT, et al. Nanoparticle-based ARV drug combinations for synergistic inhibition of cell-free and cell–cell HIV transmission. Mol Pharm. 2015;12:4363–74.
Freeling JP, Ho RJY. Anti-HIV drug particles may overcome lymphatic drug insufficiency and associated HIV persistence. Proc Natl Acad Sci U S A. 2014;111:E2512–3. https://doi.org/10.1073/pnas.1406554111.
Danaei M, Dehghankhold M, Ataei S, Davarani FH, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:57.
Madureira AN, Campos DA, Oliveira A, Sarmento O, Pintado MM, Gomes A. Insights into the protective role of solid lipid nanoparticles on rosmarinic acid bioactivity during exposure to simulated gastrointestinal conditions. Colloids Surf B: Biointerfaces. 2016;139:277–84. https://doi.org/10.1016/j.colsurfb.2015.11.039.
Poovi G, Damodharan N. Lipid nanoparticles: a challenging approach for oral delivery of BCS class-II drugs. FJPS. 2018;4:191–205. https://doi.org/10.1016/j.fjps.2018.04.001.
Das S, Chaudhary A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12:62–76. https://doi.org/10.1208/s12249-010-9563-0.
Chime SA, Onyishi IV. Lipid-based drug delivery systems (LDDS): recent advances and applications of lipids in drug delivery. Afr J Pharm Pharmacol. 2013;7(48):3034–59.
Ana B, Solinis MA, Rodriguez-Gascon A, Almeida AJ, Preat V. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomedicine. 2016;12:143–61. https://doi.org/10.1016/j.nano.2015.09.004.
Harde H, Das M, Jain S. Solid lipid nanoparticles: an oral bioavailability enhancer vehicle. Expert Opin Drug Deliv. 2011;8(11):1407–24.
Muchow M, Maincent P, Muller RH. Lipid nanoparticles with a solid matrix (SLN, NLC, LDC) for oral drug delivery. Drug Dev Ind Pharm. 2008;34:1394–405.
Cai S, Yang Q, Bagby TR, Forrest ML. Lymphatic drug delivery using engineered liposomes and solid lipid nanoparticles. Adv Drug Deliv Rev. 2011;63:901–8.
Üner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomedicine. 2007;2(3):289–300.
Kumar S, Narayan R, Ahammed V, Nayak Y, Naha A. Nayak UY, Development of ritonavir solid lipid nanoparticles by Box Behnken design for intestinal lymphatic targeting. J Drug Deliv Sci Technol. 2018. https://doi.org/10.1016/j.jddst.2017.12.014.
Savage AC, Tatham LM, Siccardi M, Scott T, Vourvahis M, Clark A, et al. Improving maraviroc oral bioavailability by formation of solid drug nanoparticles. Eur J Pharm Biopharm. 2018. https://doi.org/10.1016/j.ejpb.2018.05.015.
Desai J, Thakkar H. Darunavir-loaded lipid nanoparticles for targeting to HIV reservoirs. AAPS PharmSciTech. 2017;19:648–60. https://doi.org/10.1208/s12249-017-0876-0.
Javan F, Vatanara A, Azadmanesh K, Nabi-Meibodi M, Shakouri M. Encapsulation of ritonavir in solid lipid nanoparticles: in-vitro anti-HIV-1 activity using lentiviral particles. J Pharm Pharmacol. 2017;69(8):1002–9.
Vivek M, Jain R, Patel K, Nivsarkar M, Joshi A. Solid lipid nanoparticles (SLN) of efavirenz as lymph targeting drug delivery system: elucidation of mechanism of uptake using chylomicron flow blocking approach. Int J Pharm. 2015;495:439–46. https://doi.org/10.1016/j.ijpharm.2015.09.014.
Purvin S, Vuddanda PR, Singh SK, Jain A, Singh S. Pharmacokinetic and tissue distribution study of solid lipid nanoparticles of zidovudine in rats. J Nanotechnology. 2014;2014:1–7. https://doi.org/10.1155/2014/854018.
Ravi PR, Vats R, Dalal V, Murthy AN. A hybrid design to optimize preparation of lopinavir loaded solid lipid nanoparticles and comparative pharmacokinetic evaluation with marketed lopinavir/ritonavir coformulation. J Pharm Pharmacol. 2014;66(7):912–26.
Liptrott NJ, Giardiello M, McDonald TO, Rannard SP, Owen A. Assessment of interactions of efavirenz solid drug nanoparticles with human immunological and haematological systems. J Nanobiotechnol. 2018;16:22. https://doi.org/10.1186/s12951-018-0349-y.
Xia D, He Y, Li Q, Hu C, Huang W, Zhang Y, et al. Transport mechanism of lipid covered saquinavir pure drug nanoparticles in intestinal epithelium. J Control Release. 2017;269:159–70. https://doi.org/10.1016/j.jconrel.2017.11.012.
Raina H, Kaur S, Jindal AB. Development of efavirenz loaded solid lipid nanoparticles: risk assessment, quality-by-design (QbD) based optimisation and physicochemical characterisation. J Drug Deliv Sci Technol. 2017;39:180–91. https://doi.org/10.1016/j.jddst.2017.02.013.
Bhalekar M, Upadhaya P, Madgulkar A. Formulation and characterization of solid lipid nanoparticles for an anti-retroviral drug darunavir. Appl Nanosci. 2017;7:47–57.
Joshy KS, Sharma CP, Kalarikkal N, Sandeep K, Thomas S, Pothen LA. Evaluation of in-vitro cytotoxicity and cellular uptake efficiency of zidovudine-loaded solid lipid nanoparticles modified with aloe vera in glioma cells. Mater Sci Eng C Mater Biol Appl. 2016;66:40–50.
Dandagi PM, Patel PD, Gadad AP, Aravapalli AK. RES and brain targeting stavudine-loaded solid lipid nanoparticles for AIDS therapy. Asian J Pharm. 2012;6:116. https://doi.org/10.4103/0973-8398.102934.
Negi JS, Sharma AK, Chattopadhyay P, Ram V. Development and evaluation of glyceryl behenate based solid lipid nanoparticles (SLNs) using hot self-nanoemulsification (SNE). Technique. Arch Pharm Res. 2014;37:361–70.
McDonald TO, Giardiello M, Martin P, Siccardi M, Liptrott NJ, Smith D, et al. Antiretroviral solid drug nanoparticles with enhanced Oral bioavailability: production, characterization, and in vitro–in vivo correlation. Adv Healthcare Mater. 2014;3:400–11.
Beloqui A, Solinís MA, Rieux AD, Préat V, Gascón AR. Dextran–protamine coated nanostructured lipid carriers as mucus penetrating nanoparticles for lipophilic drugs. Int J Pharm. 2014;468:105–11.
Poonia N, Kharb R, Lather V, Pandita D. Nanostructured lipid carriers: versatile oral delivery vehicle. Future Sci OA. 2016;2:FSO135. https://doi.org/10.4155/fsoa-2016-0030.
Kasongo KW, Shegokar R, Müller RH, Walker RB. Formulation development and in vitro evaluation of didanosine-loaded nanostructured lipid carriers for the potential treatment of AIDS dementia complex. Drug Dev Ind Pharm. 2011;37(4):396–407.
Kuo YC, Chung JF. Physicochemical properties of nevirapine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Colloids Surf B Biointerfaces. 2011;83:299–306.
Beloqui A, Solinís MA, Gascón AR, Rodríguez AP, Rieux AD, Préat V. Mechanism of transport of saquinavir-loaded nanostructured lipid carriers across the intestinal barrier. J Control Release. 2013;166:115–23.
Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. Biotech. 2015;5:123–7.
Gupta H, Eral B, Hatton TA, Doyle P. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12:2826–41.
McClements DJ. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter. 2012;8:1719–29.
Manyarara TE, Khoza S, Dube A, Maponga CC. Formulation and characterization of a paediatric nanoemulsion dosage form with modified oral drug delivery system for improved dissolution rate of nevirapine. MRS Advances. 2018;3:2203–19. https://doi.org/10.1557/adv.2018.320.
Vyas TK, Shahiwala A, Amiji MM. Improved oral bioavailability and brain transport of Saquinavir upon administration in novel nanoemulsion formulations. Int J Pharm. 2008;347:93–101.
Hobson JJ, Owen A, Edwards S, Rannard SP. Branched copolymer-stabilised nanoemulsions as new candidate oral drug delivery systems. RSC Adv. 2018;8:12984–91.
Kotta S, Khan AW, Ansari SH, Sharma RK, Ali J. Anti HIV nanoemulsion formulation: optimization and in vitro-in vivo evaluation. Int J Pharm. 2014;462:129–34.
Nayak D, Boxi A, Ashe S, Thathapudi NC, Nayak B. Stavudine loaded gelatin liposomes for HIV therapy: preparation, characterization and in vitro cytotoxic evaluation. Mater Sci Eng C Mater Biol Appl. 2017;73:406–16.
Elgart A, Cherniakov I, Aldouby Y, Domb AJ, Hoffman A. Lipospheres and pro-nano lipospheres for delivery of poorly water soluble compounds. Chem Phys Lipids. 2012;165:438–53.
Lembo D, Donalisio M, Civra A, Argenziano M, Cavalli R. Nanomedicine formulations for the delivery of antiviral drugs: a promising solution for the treatment of viral infections. Expert Opin Drug Deliv. 2018;15(1):93–114. https://doi.org/10.1080/17425247.2017.1360863.
Spinks CB, Zidan AS, Khan MA, Habib MJ, Faustino PJ. Pharmaceutical characterization of novel tenofovir liposomal formulations for enhanced oral drug delivery: in vitro pharmaceutics and Caco-2 permeability investigations. Clin Pharmacol. 2017;9:29–38.
Maderkhani E, Erber A, Basnet NS, Flaten GE. Improved permeability of acyclovir: optimization of mucoadhesive liposomes using the phospholipid vesicle-based permeation assay. J Pharm Sci. 2014;103:661–8. https://doi.org/10.1002/jps.23845.
Ramana LN, Sharma S, Sethuraman S, Ranga U, Krishnan UM. Stealth anti-CD4 conjugated immunoliposomes with dual antiretroviral drugs—modern Trojan horses to combat HIV. Eur J Pharm Biopharm. 2015;89:300–11.
Ahammed V, Narayan R, Paul J, Nayak Y, Shavi GV, Nayak UY, et al. Development and in vivo evaluation of functionalized ritonavir proliposomes for lymphatic targeting. Life Sci. 2017;183:11–20.
Patel GM, Shelat PK, Lalwani AN. QbD based development of proliposome of lopinavir for improved oral bioavailability. Eur J Pharm Sci. 2016;108:50–61. https://doi.org/10.1016/j.ejps.2016.08.057.
Kallakunta VR, Bandari S, Jukanti R, Veerareddy PR. Oral self emulsifying powder of lercanidipine hydrochloride: formulation and evaluation. Powder Technol. 2012;221:375–82.
Makadia HA, Bhatt AY, Parmar RB, Paun JS, Tank HM. Self-nano emulsifying drug delivery system (SNEDDS): future aspects. Asian J Pharm Res. 2013;3:21–7.
Mooter GVD, Thia TD, Speybroeck MV, Barillaro V, Martens J, Annaert P, et al. Formulate-ability of ten compounds with different physicochemical profiles in SMEDDS. Eur J Pharm Sci. 2009;38:479–88.
Selvam RP, Kulkarni PK. Design and evaluation of self nanoemulsifying systems for poorly water soluble HIV drug. J Pharma Sci Tech. 2014;4(1):23–8.
Selvam RP, Kulkarni PK, Dixit M. Preparation and evaluation of self-nanoemulsifying formulation of efavirenz. Ind J Pharm Edu Res. 2013;47(1):47–54.
Patel GM, Shelat PK, Lalwani AN. Statistical modeling, optimization and characterization of solid self-nanoemulsifying drug delivery system of lopinavir using design of experiment. Drug Deliv. 2016;23(8):3027–42.
Singh G, Pai RS. Optimized self-nanoemulsifying drug delivery system of atazanavir with enhanced oral bioavailability: in vitro/in vivo characterization. Expert Opin Drug Deliv. 2014;11:1023–32. https://doi.org/10.1517/17425247.2014.913566.
Senapati PC, Sahoo SK, Sahu AN. Mixed surfactant based (SNEDDS) self-nanoemulsifying drug delivery system presenting efavirenz for enhancement of oral bioavailability. Biomed Pharmacother. 2016;80:42–51.
Kamble RN, Mehta PP, Kumar A. Efavirenz self-nano-emulsifying drug delivery system: in vitro and in vivo evaluation. AAPS PharmSciTech. 2016;17(5):1240–7.
Obitte NC, Rohan LC, Adeyeye CM, Parniak MA, Esimone CO. The utility of self-emulsifying oil formulation to improve the poor solubility of the anti HIV drug CSIC. AIDS Res Ther. 2013;10(14):1–9.
Garg B, Katare OP, Beg S, Lohan S, Singh B. Systematic development of solid self-nanoemulsifying oily formulations (S-SNEOFs) for enhancing the oral bioavailability and intestinal lymphatic uptake of lopinavir. Colloids Surf B Biointerfaces. 2016;141:611–22.
Garg B, Beg S, Kaur R, Kumar R, Katare OP, Singh B. Long-chain triglycerides-based self-nanoemulsifying oily formulations (SNEOFs) of darunavir with improved lymphatic targeting potential. J Drug Target. 2017;26:252–66. https://doi.org/10.1080/1061186X.2017.1365875.
Sahoo SK, Dash GS, Biswal S, Biswal PK, Senapati PC. Fabrication and evaluation of self-nanoemulsifying oil formulations (SNEOFs) of Efavirenz. J Dispers Sci Technol. 2018:1–12. https://doi.org/10.1080/01932691.2018.1472008.
Cavalcanti SMT, Nunes C, Lima SAC, Soares-Sobrinho JL, Reis S. Multiple lipid nanoparticles (MLN), a new generation of lipid nanoparticles for drug delivery systems: lamivudine-MLN experimental design. Pharm Res. 2017;34:1204–16.
Varshosaz J, Taymouri S, Ali J, Alizadeh A. Efavirenz oral delivery via nanocapsules: formulation, optimisation, and ex-vivo gut permeation study. IET Nanobiotechnol. 2018;12(6):795–806.
Kamboj S, Sethi S, Rana V. Lipid based delivery of Efavirenz: an answer to its erratic absorption and food effect. Eur J Pharm Sci. 2018;123:199–216. https://doi.org/10.1016/j.ejps.2018.07.037.
Jindal AB, Bachhav SS, Devarajan PV. In situ hybrid nano drug delivery system (IHN-DDS) of antiretroviral drug for simultaneous targeting to multiple viral reservoirs: an in vivo proof of concept. Int J Pharm. 2017;521:196–203. https://doi.org/10.1016/j.ijpharm.2017.02.024.
Evrim AT, Özge I, Tamer B. Studies on transdermal delivery enhancement of zidovudine. AAPS PharmSciTech. 2009;10(1):88–97.
Sharma P, Garg S. Pure drug and polymer based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugs. Adv Drug Deliv Rev. 2010;62:491–502.
Roy U, Rodríguez J, Barber P, das Neves J, Sarmento B, Nair M. The potential of HIV-1 nanotherapeutics: from in vitro studies to clinical trials. Nanomedicine. 2015;10(24):3597–609.
Shao J, Kraft JC, Li B, Yu J, Freeling J, Koehn J, et al. Nanodrug formulations to enhance HIV drug exposure in lymphoid tissues and cells: clinical significance and potential impact on treatment and eradication of HIV/AIDS. Nanomedicine. 2016;11(5):545–64.
Tatham LM, Rannard SP, Owen A. Nanoformulation strategies for the enhanced oral bioavailability of antiretroviral therapeutics. Ther Deliv. 2015;6(4):469–90.
Hafner A, Lovriɛ J, Lakoš GP, Pepiɛ I. Nanotherapeutics in the EU: an overview on current state and future directions. Int J Nanomedicine. 2014;9:1005–23.
Acknowledgements
The authors acknowledge Jamia Hamdard, New Delhi, India, for providing Jamia Hamdard Silver Jubilee Research Fellowship to the first author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Additional information
Guest Editor: Sanyog Jain
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nabi, B., Rehman, S., Baboota, S. et al. Insights on Oral Drug Delivery of Lipid Nanocarriers: a Win-Win Solution for Augmenting Bioavailability of Antiretroviral Drugs. AAPS PharmSciTech 20, 60 (2019). https://doi.org/10.1208/s12249-018-1284-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-018-1284-9