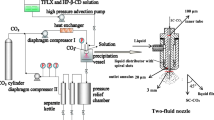
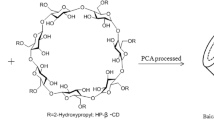
Abstract
The objective of this study was to determine the pharmacokinetic parameters of miconazole after oral administration of a miconazole/hydroxypropyl-γ-cyclodextrin(HPγCD)/ L-tartaric acid inclusion complex produced by supercritical carbon dioxide processing. The pharmacokinetics of the miconazole ternary complex (CPLX), of the corresponding physical mixture (PHYS), and of miconazole alone (MICO) were compared after oral administration. Six mixed-breed pigs received each formulation as a single dose (10 mg miconazole/kg) in a crossover design. Miconazole plasma concentrations were determined by a high-performance liquid chromatography method. Preliminary in vitro dissolution data showed that CPLX exhibits a faster and higher dissolution rate than either PHYS or MICO. Following CPLX oral administration, mean area under the plasma concentration curve (AUC0−∞) for miconazole was 95.0±55.8 μg/min/mL, with the peak plasma concentration (Cmax 0.59±0.39 μg/mL) at 19.30 minutes. The AUC0−∞ and Cmax values were significantly higher than those after oral administration of PHYS (AUC0−∞ 38.5±12.7 μg/min/mL and Cmax 0.24±0.08 μg/mL;P<.1) and of MICO (AUC0−∞ 24.1±14.0 μg/min/mL and Cmax 0.1±0.05 μg/mL;P<.1). There were also significant differences between PHYS and MICO (P<.1). The results of the study indicate that CPLX shows improved dissolution properties and a higher relative oral bioavailability compared with PHYS and MICO.
Similar content being viewed by others
References
Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation.Eur J Cancer. 2001;37:1590–1598.
Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization.J Pharm Sci. 1996;85:1017–1025.
Jacobsen J, Bjerregaard S, Pedersen M. Cyclodextrin inclusion complexes of antimycotics intended to act in the oral cavity-drug supersaturation, toxicity on TR146 cells and release from a delivery system.Eur J Pharm Biopharm. 1999;48:217–224.
Mura P, Liguori A, Bramanti G, Bettinetti GP, Campisi E, Faggi E. Improvement of dissolution properties and microbiological activity of miconazole and econazole by cyclodextrin complexation.Eur J Pharm Biopharm. 1992;38:119–123.
Pedersen M. Isolation and antimycotic effect of a genuine miconazole β-cyclodextrin complex.Eur J Pharm Biopharm. 1994;40:19–23.
Piel G, Evrard B, Fillet M, Llabres G, Delattre L. Development of a non-surfactant parenteral formulation of miconazole by the use of cyclodextrins.Int J Pharm. 1998;169:15–22.
Rajewski RA, Stella VJ. Pharmaceutical applications of cyclodextrins. II. In vivo drug delivery.J Pharm Sci. 1996;85:1142–1165.
Tenjarla S, Puranajoti P, Kasina R, Mandal T. Preparation, characterization, and evaluation of miconazole-cyclodextrin complexes for improved oral and topical delivery.J Pharm Sci. 1998;87:425–429.
Hostetler JS, Hanson LH, Stevens DA. Effect of cyclodextrin on the pharmacology of antifungal oral azoles.Antimicrob Agents Chem. 1992;36:477–480.
Van Hees T, Piel G, Evrard B, Otte X, Thunus L, Delattre L. Application of supercritical fluid carbon dioxide for the preparation of a piroxicam-β-cyclodextrin inclusion compound.Pharm Res. 1999;16:1864–1870.
Charoenchaitrakool M, Dehghani F, Foster NR. Utilization of supercritical carbon dioxide for complex formation of ibuprofen and methyl-β-cyclodextrin.Int J Pharm. 2002;239:103–112.
Lai S, Locci E, Piras A, Porcedda S, Lai A, Marongiu B. Imazalil-cyclomaltoheptaose (β-cyclodextrin) inclusion complex: preparation by supercritical carbon dioxide and13C CPMAS and1H NMR characterization.Carbohydr Res. 2003;338:2227–2232.
Barillaro V, Bertholet P, Henry de Hassonville S, et al. Effect of acidic ternary compounds on the formation of miconazole/cyclodextrin inclusion complexes by means of supercritical carbon dioxide.J Pharm Pharm Sci. 2004;7:378–388.
Kararli TT. Comparison of the gatrointestinal anatomy, physiology and biochemistry of humans and commonly used laboratory animals.Biopharm Drug Dispos. 1995;16:351–380.
Davis SS, Illum L, Hinchcliffe M. Gastrointestinal transit of dosage forms in the pig.J Pharm Pharmacol. 2001;53:33–39.
Larsen F, Jensen BH, Olesen HP, Larsen P. Multiple oral administration of a ketoprofen-dextran ester prodrug in pigs: assessment of gastrointestinal bioavailability by deconvolution.Pharm Res. 1992;9:915–919.
Hildebrand H, McDonald FM, Windt-Hanke E. Characterization of oral sustained release preparations of iloprost in the pig model by plasma level monitoring.Prostaglandins. 1991;41:473–486.
Piel G, Evrard B, Van Hees T, Delattre L. Comparison of the IV pharmacokinetics in sheep of miconazole-cyclodextrin solutions and a micellar solution.Int J Pharm. 1999;180:41–45.
US Food and Drug Administration Center for Drug Evaluation and Research.Bioanalytical Method Validation. Rockville, Maryland: FDA Office of Training and Communication, Division of Communication Management, Drug Information Branch, 2001.
Van Hees T, Piel G, Henry de Hassonville S, Evrard B, Delattre L. Determination of the free/included piroxicam ratio in cyclodextrin complexes: comparison between UV spectroscopy and differential scanning calorimetry.Eur J Pharm Sci. 2002;15:347–353.
Stella VJ, Rao VM, Zannou EA, Zia V. Mechanisms of drug release from cyclodextrin complexes.Adv Drug Deliv Rev. 1999;36:3–16.
Uekama K, Hirayama F, Irie T. Cyclodextrin drug carrier systems.Chem Rev. 1998;98:2045–2076.
Savolainen J, Jarvinen K, Matilainen L, Jarvinen T. Improved dissolution and bioavailability of phenytoin by sulfobutylether-β-cyclodextrin ((SBE) 7m-β-CD) and hydroxypropyl-β-cyclodextrin (HP-β-CD) complexation.Int J Pharm. 1998;165:69–78.
Koester LS, Bertuol JB, Groch KR, et al. Bioavailability of carbamazepine: β-cyclodextrin complex in beagle dogs from hydroxypropylmethylcellulose matrix tablets.Eur J Pharm Sci. 2004;22:201–207.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: August 18, 2005
Rights and permissions
About this article
Cite this article
Barillaro, V., Evrard, B., Delattre, L. et al. Oral bioavailability in pigs of a miconazole/Hydroxypropyl-γ-cyclodextrin/ L-tataric acid inclusion complex produced by supercritical carbon dioxide processing. AAPS J 7, 16 (2005). https://doi.org/10.1208/aapsj070116
Received:
Accepted:
DOI: https://doi.org/10.1208/aapsj070116