Abstract
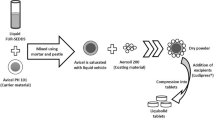
The purpose of this study was to combine the advantages of self-nanoemulsifying drug delivery systems and tablets as a conventional dosage form emphasizing the excipients’ effect on the development of a new dosage form. Systems composed of HCO-40, Transcutol® HP, and medium-chain triglyceride were prepared. Essential properties of the prepared systems regarding carvedilol solubility, a model drug, and self-emulsification time were determined. In order to optimize self-nanoemulsifying drug delivery systems (SNEDDS), formulation dispersion–drug precipitation test was performed in the absence and presence of cellulosic polymers. Furthermore, SNEDDS was loaded onto liquisolid powders. P-glycoprotein (P-gp) activity of the selected SNEDDS was tested using HCT-116 cells. Carvedilol showed acceptable solubility in the selected excipients. It also demonstrated improvement in the stability upon dilution with aqueous media in the presence of cellulosic polymers. Use of granulated silicon dioxide improved the physical properties of liquisolid powders containing SNEDDS. It improved the compressibility of the selected powders and the tested SNEDDS showed marked P-gp inhibition activity. Prepared self-nanoemulsifying tablet produced acceptable properties of immediate-release dosage forms and expected to increase the bioavailability of carvedilol.







Similar content being viewed by others

References
I. Weuts, D. Kempen, A. Decorte, G. Verreck, J. Peeters, M. Brewster, and G. Van den Mooter. Phase behaviour analysis of solid dispersions of loperamide and two structurally related compounds with the polymers PVP-K30 and PVP-VA64. Eur. J. Pharm. Sci. 22:375–385 (2004).
H. O. Ammar, H. A. Salama, M. Ghorab, and A. A. Mahmoud. Implication of inclusion complexation of glimepiride in cyclodextrin-polymer systems on its dissolution, stability and therapeutic efficacy. Int. J. Pharm. 320:53–57 (2006).
G. Muhrer, U. Meier, F. Fusaro, S. Albano, and M. Mazzotti. Use of compressed gas precipitation to enhance the dissolution behavior of a poorly water-soluble drug: generation of drug microparticles and drug-polymer solid dispersions. Int. J. Pharm. 308:69–83 (2006).
J. M. Odeberg, P. Kaufmann, K. G. Kroon, and P. Hoglund. Lipid drug delivery and rational formulation design for lipophilic drugs with low oral bioavailability, applied to cyclosporine. Eur. J. Pharm. Sci. 20:375–382 (2003).
T. Gershanik, and S. Benita. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur. J. Pharm. Biopharm. 50:179–188 (2000).
R. N. Gursoy, and S. Benita. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 58:173–182 (2004).
C. W. Pouton. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 29:278–287 (2006).
C. W. Pouton. Formulation of self-emulsifying drug delivery systems. Adv. Drug Deliv. Rev. 25:47–58 (1997).
M. Newton, J. Petersson, F. Podczeck, A. Clarke, and S. Booth. The influence of formulation variables on the properties of pellets containing a self-emulsifying mixture. J. Pharm. Sci. 90:987–995 (2001).
C. Tuleu, M. Newton, J. Rose, D. Euler, R. Saklatvala, A. Clarke, and S. Booth. Comparative bioavailability study in dogs of a self-emulsifying formulation of progesterone presented in a pellet and liquid form compared with an aqueous suspension of progesterone. J. Pharm. Sci. 93:1495–1502 (2004).
E. Franceschinis, D. Voinovich, M. Grassi, B. Perissutti, J. Filipovic-Grcic, A. Martinac, and F. Meriani-Merlo. Self-emulsifying pellets prepared by wet granulation in high-shear mixer: influence of formulation variables and preliminary study on the in vitro absorption. Int. J. Pharm. 291:87–97 (2005).
J. M. Newton, M. Bazzigialuppi, F. Podczeck, S. Booth, and A. Clarke. The rheological properties of self-emulsifying systems, water and microcrystalline cellulose. Eur. J. Pharm. Sci. 26:176–183 (2005).
J. M. Newton, A. Godinho, A. P. Clarke, and S. W. Booth. Formulation variables on pellets containing self-emulsifying systems. Pharm. Technol. Eur. 17:29–32 (2005).
S. Nazzal, M. Nutan, A. Palamakula, R. Shah, A. A. Zaghloul, and M. A. Khan. Optimization of a self-nanoemulsified tablet dosage form of ubiquinone using response surface methodology: effect of formulation ingredients. Int. J. Pharm. 240:103–114 (2002).
N. A. Kasim, M. Whitehouse, C. Ramachandran, M. Bermejo, H. Lennernas, A. S. Hussain, H. E. Junginger, S. A. Stavchansky, K. K. Midha, V. P. Shah, and G. L. Amidon. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol. Pharm. 1:85–96 (2004).
R. L. Talbert. Pharmacokinetics and pharmacodynamics of beta blockers in heart failure. Heart Fail. Rev. 9:131–137 (2004).
W. H. Frishman. Carvedilol. Drug Therapy. 339:1759–1765 (1998).
T. Giessmann, C. Modess, U. Hecker, M. Zschiesche, P. Dazert, C. Kunert-Keil, R. Warzok, G. Engel, W. Weitschies, I. Cascorbi, H. K. Kroemer, and W. Siegmund. CYP2D6 genotype and induction of intestinal drug transporters by rifampin predict presystemic clearance of carvedilol in healthy subjects. Clin. Pharmacol. Ther. 75:213–222 (2004).
X. Wen, F. Tan, Z. Jing, and Z. Liu. Preparation and study the 1:2 inclusion complex of carvedilol with β-cyclodextrin. J. Pharm. Biomed. Anal. 34:517–523 (2004).
K. Bogman, F. Erne-Brand, J. Alsenz, and J. Drewe. The role of surfactants in the reversal of active transport mediated by multidrug resistance proteins. J. Pharm. Sci. 92:1250–1261 (2003).
S. Yang, R. N. Gursoy, G. Lambert, and S. Benita. Enhanced oral absorption of paclitaxel in a novel self-microemulsifying drug delivery system with or without concomitant use of P-glycoprotein inhibitors. Pharm. Res. 21:261–270 (2004).
H. Shen, and M. Zhong. Preparation and evaluation of self-microemulsifying drug delivery systems (SMEDDS) containing atorvastatin. J. Pharm. Pharmacol. 58:1183–1191 (2006).
T. Gershanik, E. Haltner, C. M. Lehr, and S. Benita. Charge-dependent interaction of self-emulsifying oil formulations with Caco-2 cells monolayers: binding, effects on barrier function and cytotoxicity. Int. J. Pharm. 211:29–36 (2000).
Y. Tayrouz, R. Ding, J. Burhenne, K. D. Riedel, J. Weiss, T. Hoppe-Tichy, W. E. Haefeli, and G. Mikus. Pharmacokinetic and pharmaceutic interaction between digoxin and Cremophor RH40. Clin. Pharmacol. Ther. 73:397–405 (2003).
C. J. Porter, and W. N. Charman. In vitro assessment of oral lipid based formulations. Adv. Drug. Deliv. Rev. 1Suppl 50:S127–S147 (2001).
G. Cornaire, J. Woodley, P. Hermann, A. Cloarec, C. Arellano, and G. Houin. Impact of excipients on the absorption of P-glycoprotein substrates in vitro and in vivo. Int. J. Pharm. 278:119–131 (2004).
M. Grovea, A. Müllertzb, J. L. Nielsenc, and P. Pedersen. Bioavailability of seocalcitol II: development and characterisation of self-microemulsifying drug delivery systems (SMEDDS) for oral administration containing medium and long chain triglycerides. Eur. J. Pharm. Sci. 28:233–242 (2006).
S. Cui, C. Zhao, X. Tang, D. Chen, and Z. He. Study on the bioavailability of puerarin from Pueraria lobata isoflavone self-microemulsifying drug-delivery systems and tablets in rabbits by liquid chromatography–mass spectrometry. Biomed. Chromatogr. 19:375–378 (2005).
J. Y. Hong, J. K. Kim, Y. K. Song, J. S. Park, and C. K. Kim. A new self-emulsifying formulation of itraconazole with improved dissolution and oral absorption. J. Control Release. 110:332–338 (2006).
P. Gao, B. D. Rush, W. P. Pfund, T. Huang, J. M. Bauer, W. Morozowich, M. S. Kuo, and M. J. Hageman. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. J. Pharm. Sci. 92:2386–2398 (2003).
S.-M. Khoo, A. J. Humberstone, C. J. H. Porter, G. A. Edwards, and W. N. Charman. Formulation design and bioavailability assessment of lipidic self-emulsifying formulations of halofantrine. Int. J. Pharm. 167:155–164 (1998).
B. K. Kang, J. S. Lee, S. K. Chon, S. Y. Jeong, S. H. Yuk, G. Khang, H. B. Lee, and S. H. Cho. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 274:65–73 (2004).
P. Li, A. Ghosh, R. F. Wagner, S. Krill, Y. M. Joshi, and A. T. Serajuddin. Effect of combined use of nonionic surfactant on formation of oil-in-water microemulsions. Int. J. Pharm. 288:27–34 (2005).
N. Hasan, S. H. Moss, and C. W. Pouton. Through, C. W. Pouton Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 29:278–287 (2006).
S. Spireas, S. Sadu, and R. Grover. In vitro release evaluation of hydrocortisone liquisolid tablets. J. Pharm. Sci. 87:867–872 (1998).
J. N. Staniforth. PharmaceuticsIn M. E. Aulton (ed.), The science of dosage form design, Churchill, Livingstone, 1988, p. 610.
P. J. Sinko (ed.). Martin’s physical pharmacy and biopharmaceutical sciences, 5th ed., Lippincott Williams &Wilkins, Philadelphia, 2006, p. 558.
G. Abdelbary, P. Prinderre, C. Eouani, J. Joachim, J. P. Reynier, and P. Piccerelle. The preparation of orally disintegrating tablets using a hydrophilic waxy binder. Int. J. Pharm. 278:423–433 (2004).
M.-E. Chateau, L. Galet, Y. Soudais, and J. Fages. Processing a detergent powder formulation: direct compression, and high shear wet granulation followed by compression. Powder Technol. 157:191–198 (2005).
British Pharmacopoeia Commission. British pharmacopoeia 2002, Her Majesty's Stationary Office, London, 2002.
Council of Europe. European pharmacopoeia, 5th ed., European Directorate for the Quality of Medicines, Strasbourg, 2005.
J. C. Smith, G. Tarocco, F. Merazz, and U. Salzmann. Are generic formulations of carvedilol of inferior pharmaceutical quality compared with the branded formulation? Curr. Med. Res. Opin. 22:709–720 (2006).
US Pharmacopoeia Convention. United States Pharmacopoeia, 3rd ed., US Pharmacopoeia Convention, Rockville, 2002.
M. B. Hansen, S. E. Nielsen, and K. Berg. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods. 12:203–210 (1989).
S.-W. Wang, J. Monagle, C. McNulty, D. Putnam, and H. Chen. Determination of P-glycoprotein inhibition by excipients and their combinations using an integrated high-throughput process. J. Pharm. Sci. 93:2755–2767 (2004).
T. C. Chou, and P. Talalay. Quantitative analysis of dose–effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme. Regul. 22:27–55 (1984).
T. C. Chou, and P. Talaly. A simple generalized equation for the analysis of multiple inhibitions of Michaelis–Menten kinetic systems. J. Biol. Chem. 252:6438–6442 (1977).
M. Y. Levy, and S. Benita. Drug release from submicronized o/w emulsion: a new in vitro kinetic evaluation model. Int. J. Pharm. 66:29–37 (1990).
E. S. Kostewicz, M. Wunderlich, U. Brauns, R. Becker, T. Bock, and J. B. Dressman. Predicting the precipitation of poorly soluble weak bases upon entry in the small intestine. J. Pharm. Pharmacol. 56:43–51 (2004).
K. Nolte, G. Backfisch, and R. Neidlein. In vitro absorption studies with carvedilol using a new model with porcine intestine called BM-RIMO (Boehringer–Mannheim ring model). Arzneimittelforschung. 49:745–749 (1999).
S. Stegemanna, F. Leveillerb, D. Franchic, H. de Jongd, and H. Lindén. When poor solubility becomes an issue: from early stage to proof of concept. Eur. J. Pharm. Sci. 31:249–261 (2007).
E. Galia, E. Nicolaides, D. Horter, R. Lobenberg, C. Reppas, and J. B. Dressman. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm. Res. 15:698–705 (1998).
K. Itoh, Y. Tozuka, T. Oguchi, and K. Yamamoto. Improvement of physicochemical properties of N-4472 part I formulation design by using self-microemulsifying system. Int. J. Pharm. 238:153–160 (2002).
P. Gao, M. E. Guyton, T. Huang, J. M. Bauer, K. J. Stefanski, and Q. Lu. Enhanced oral bioavailability of a poorly water soluble drug PNU-91325 by supersaturable formulations. Drug Dev. Ind. Pharm. 30:221–229 (2004).
L. Cisneros-Zevallos, and J. M. Krochta. Dependence of coating thickness on viscosity of coating solution applied to fruits and vegetables by dipping method. J. Food Sci. 68:503–510 (2003).
S. M. Grillo, R. M. Steffenino, D. C. Kunkle, and K. Saraceni. Aqueous maltodextrin and cellulosic polymer film coatings. WO 91/15548. (1991).
S. Naruenartwongsakul, M. S. Chinnan, S. Bhumiratana, and T. Yoovidhya. Pasting characteristics of wheat flour-based batters containing cellulose ethers. Lebensm. Wiss. Technol. 37:489–495 (2004).
L. Wei, P. Sun, S. Nie, and W. Pan. Preparation and evaluation of SEDDS and SMEDDS containing carvedilol. Drug Dev. Ind. Pharm. 31:785–794 (2005).
Acknowledgement
I am very grateful for the Sasol, Gattefosse, and Colorcon companies and Mohamed H. AbouGhaly for providing the required chemicals for my research. I am grateful to Assoc. Prof. Amira M. Gamal-Eldeen, Associate Professor of Genetic Engineering and Biotechnology Division, National Research Center, for her kind help in the toxicological experimental work in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahmoud, E.A., Bendas, E.R. & Mohamed, M.I. Preparation and Evaluation of Self-nanoemulsifying Tablets of Carvedilol. AAPS PharmSciTech 10, 183–192 (2009). https://doi.org/10.1208/s12249-009-9192-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-009-9192-7