Abstract
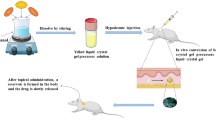
Gastrodin is the major bioactive constituent of the traditional Chinese drug “Tianma.” It is used in the treatment of some nervous system diseases and can be transported to the brain via intranasal administration. In the current paper, the development of a novel ion-activated in situ gelling system for the nasal delivery of gastrodin is discussed. An in situ perfusion model was used to determine the absorption-rate constant of gastrodin through rat nasal mucosa. The optimal formulation was determined by measuring the critical cation concentration, anti-dilution capacity, gel expansion coefficient, water-holding capacity, and adhesive capacity. The best formulation consisted of 10% gastrodin, 0.5% deacetylated gellan gum as the gelatinizer, and 0.03% ethylparaben as the preservative. The rheological properties of gastrodin nasal in situ gels were also investigated. The viscosity and elasticity sharply increased at temperatures below 25°C. When physiological concentrations of cations were added into the preparation, the mixture gelled into a semi-solid. The results of an accelerated stability test show that gastrodin nasal in situ gels can be stable for more than 2 years. Mucociliary toxicity was evaluated using the in situ toad palate model and the rat nasal mucociliary method; both models demonstrated no measurable ciliotoxicity. Pharmacodynamic studies suggest that similar acesodyne and sedative effects were induced following intranasal administration of 50 mg/kg gastrodin nasal in situ gels or oral administration of 100 mg/kg gastrodin solution. The in situ gel preparation is a safe and effective nasal delivery system for gastrodin.










Similar content being viewed by others
REFERENCES
Ojemann LM, Nelson WL, Shin DS, Rowe AO, Buchanan RA. Tian ma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy Behav. 2006;8:376–83.
Zhao H. Advance in clinical application of gastrodin. Strait Pharm J. 2009;21:29–31.
Zeng X, Zhang Y, Zhang S, Zheng X. A microdialysis study of effects of gastrodin on neurochemical changes in the ischemic/reperfused rat cerebral hippocampus. Biol Pharm Bull. 2007;30:801–4.
Guo Z, Tan T, Zhong Y, Wu C. Study of the mechanism of gastrodin and derivatives of gastrodigenin. J West Chin Univ Med Sci. 1991;22:79–82.
Wang Q, Chen G, Zeng S. Pharmacokinetics of Gastrodin in rat plasma and CSF after i.n. and i.v. Int J Pharm. 2007;341:20–5.
Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11:1–18.
Watts P, Smith A. PecSys: in situ gelling system for optimised nasal drug delivery. Expert Opin Drug Deliv. 2009;6:543–52.
Nagarwal RC, Pandit JK. Phase transition system: novel oral in-situ gel. Curr Drug Deliv. 2008;5:282–9.
Jansson B, Hagerstrom H, Fransen N, Edsman K, Bjork E. The influence of gellan gum on the transfer of fluorescein dextran across rat nasal epithelium in vivo. Eur J Pharm Biopharm. 2005;59:557–64.
Balasubramaniam J, Kant S, Pandit JK. In vitro and in vivo evaluation of the Gelrite gellan gum-based ocular delivery system for indomethacin. Acta Pharm. 2003;53:251–61.
Kubo W, Miyazaki S, Attwood D. Oral sustained delivery of paracetamol from in situ-gelling gellan and sodium alginate formulations. Int J Pharm. 2003;258:55–64.
Narayana RC, Harish NM, Gulzar AM, Prabhakara P, Singh AK, Subrahmanyam EV. Formulation and in vitro evaluation of in situ gels containing secnidazole for vaginitis. Yakugaku Zasshi. 2009;129:569–74.
Funami T, Noda S, Nakauma M, Ishihara S, Takahashi R, Al-Assaf S, et al. Molecular structures of gellan gum imaged with atomic force microscopy in relation to the rheological behavior in aqueous systems in the presence or absence of various cations. J Agric Food Chem. 2008;56:8609–18.
Cao SL, Ren XW, Zhang QZ, Chen E, Xu F, Chen J, et al. In situ gel based on gellan gum as new carrier for nasal administration of mometasone furoate. Int J Pharm. 2009;365:109–15.
Lorin MI, Gaerlan PF, Mandel ID. Quantitative composition of nasal secretions in normal subjects. J Lab Clin Med. 1972;80:275–81.
Mei D, Mao S, Sun W, Wang Y, Kissel T. Effect of chitosan structure properties and molecular weight on the intranasal absorption of tetramethylpyrazine phosphate in rats. Eur J Pharm Biopharm. 2008;70:874–81.
Hirai S, Yashiki T, Matsuzawa T, Mima H. Absorption of drugs from the nasal mucosa of rat. Int J Pharm. 1981;7:317–25.
Lu Y, Chen X, Du S, Wu Q, Yao Z, Zhai Y. The in situ and in vivo study on enhancing effect of borneol in nasal absorption of Geniposide in rats. Arch Pharm Res. 2010;33:691–6.
Cai Z, Hou S, Li Y, Zhao B, Yang Z, Xu S, et al. Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration. J Drug Target. 2008;16:178–84.
Tao T, Zhao Y, Yue P, Dong WX, Chen QH. Preparation of huperzine A nasal in situ gel and evaluation of its brain targeting following intranasal administration. Acta Pharm Sin. 2006;41:1104–10.
Guinee TP, Feeney EP, Auty MA, Fox PF. Effect of pH and calcium concentration on some textural and functional properties of mozzarella cheese. J Dairy Sci. 2002;85:1655–69.
Asai K, Morishita M, Katsuta H, Hosoda S, Shinomiya K, Noro M, et al. The effects of water-soluble cyclodextrins on the histological integrity of the rat nasal mucosa. Int J Pharm. 2002;246:25–35.
Jiang XG, Lu X, Cui JB, Qiu L, Xi NZ. Studies on octanol-water partition coefficient and nasal drug absorption. Acta Pharm Sin. 1997;32:458–60.
Illum L. Nasal drug delivery—possibilities, problems and solutions. J Control Release. 2003;87:187–98.
Choi HG, Jung JH, Ryu JM, Yoon SJ, Oh YK, Kim CK. Development of in situ-gelling and mucoadhesive acetaminophen liquid suppository. Int J Pharm. 1998;165:33–44.
Wei G, Xu H, Ding PT, Li SM, Zheng JM. Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. J Control Release. 2002;83:65–74.
Chung H, Dong H. Synthesis and characterisation of pluronic grafted chitosan copolymer as a novel injectable biomaterial. Curr Appl Phys. 2005;5:485–8.
Mao R, Tang J, Swanson BG. Water holding capacity and microstructure of gellan gels. Carbohydr Polym. 2001;46:365–71.
Lim ST, Martin GP, Berry DJ, Brown MB. Preparation and evaluation of the in vitro drug release properties and mucoadhesion of novel microspheres of hyaluronic acid and chitosan. J Control Release. 2000;66:281–92.
Edsman K, Carlfors J, Harju K. Rheological evaluation and ocular contact time of some carbomer gels for ophthalmic use. Int J Pharm. 1996;137:233–41.
Shusterman D. Toxicology of nasal irritants. Curr Allergy Asthma Rep. 2003;3:258–65.
Merkus FW, Verhoef JC, Schipper NG, Marttin E. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev. 1998;29:13–38.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (no. 30902009) and Guangdong Natural Science Foundation (no. 10451008901004959).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, Z., Song, X., Sun, F. et al. Formulation and Evaluation of In Situ Gelling Systems for Intranasal Administration of Gastrodin. AAPS PharmSciTech 12, 1102–1109 (2011). https://doi.org/10.1208/s12249-011-9678-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-011-9678-y