Abstract
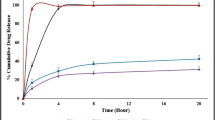
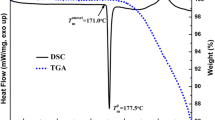
Sustained-release matrix tablets based on Eudragit RL and RS were manufactured by injection moulding. The influence of process temperature; matrix composition; drug load, plasticizer level; and salt form of metoprolol: tartrate (MPT), fumarate (MPF) and succinate (MPS) on ease of processing and drug release were evaluated. Formulations composed of 70/30% Eudragit RL/MPT showed the fastest drug release, substituting part of Eudragit RL by RS resulted in slower drug release, all following first-order release kinetics. Drug load only affected drug release of matrices composed of Eudragit RS: a higher MPT concentration yielded faster release rates. Adding triethyl citrate enhanced the processability, but was detrimental to long-term stability. The process temperature and plasticizer level had no effect on drug release, whereas metoprolol salt form significantly influenced release properties. The moulded tablets had a low porosity and a smooth surface morphology. A plasticizing effect of MPT, MPS and MPF on Eudragit RS and Eudragit RL was observed via DSC and DMA. Solubility parameter assessment, thermal analysis and X-ray diffraction demonstrated the formation of a solid solution immediately after production, in which H-bonds were formed between metoprolol and Eudragit as evidenced by near-infrared spectroscopy. However, high drug loadings of MPS and MPF showed a tendency to recrystallise during storage. The in vivo performance of injection-moulded tablets was strongly dependent upon drug loading.









Similar content being viewed by others
REFERENCES
Quinten T, De Beer T, Remon JP, Vervaet C. Overview of injection molding as a manufacturing technique for pharmaceutical application. In: Kauffer PH, editor. Injection molding: process, design and application. New York: Nova; 2011. p. 1–42.
Cheng L, Guo S, Wu W. Characterization and in vitro release of praziquantel from poly(ε-caprolactone) implants. Int J Pharm. 2009;377:112–9.
Zema L, Loreti G, Melocchi A, Maroni A, Gazzania A. Injection molding and its application to drug delivery. J Control Release. 2012;159:324–31.
Quinten T, De Beer T, Onofre FO, Mendez-Montealvo G, Wang YJ, Remon JP, et al. Sustained-release and swelling characteristics of xanthan gum/ethylcellulose-based injection moulded matrix tablets: in vitro and in vivo evaluation. J Pharm Sci. 2011;100:2858–70.
Quinten T, De Beer T, Vervaet C, Remon JP. Evaluation of injection moulding as a pharmaceutical technology to produce matrix tablets. Eur J Pharm Biopharm. 2009;71:145–54.
Quinten T, Gonissen Y, Cnudde V, Masschaele B, Van Hoorebeke L, Remon JP, et al. Development of injection moulded matrix tablets based on mixtures of ethylcellulose and low-substituted hydroxypropylcellulose. Eur J Pharm Sci. 2009;37:207–16.
Quinten T, De Beer T, Almeida A, Vlassenbroeck J, Van Hoorebeke L, Remon JP, et al. Development and evaluation of injection moulded sustained release tablets containing ethylcellulose and polyethylene oxide. Drug Dev Ind Pharm. 2011;37:149–59.
Andrews GP, Abudiak OA, Jones DS. Physicochemical characterisation of hot melt extruded bicalutamide-polyvinylpyrrolidone solid dispersions. J Pharm Sci. 2010;99:1322–35.
Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60.
Schilling SU, Bruce CD, Shah NH, Malick AW, McGinity JW. Citric acid monohydrate as a release-modifying agent in melt extruded matrix tablets. Int J Pharm. 2008;361:158–68.
Zhu YC, Shah NH, Malick AW, Infeld MH, McGinity JW. Solid-state plasticization of an acrylic polymer with chlorpheniramine maleate and triethyl citrate. Int J Pharm. 2002;241:301–10.
Gordon S, Taylor JS. Ideal copolymers and the second-order transitions of synthetic rubbers. I Non-crystalline copolymers. J Appl Chem. 1952;2:493–500.
Suhma R, Boyer RF. General relation involving the glass transition temperature and coefficient of expansion of polymers. J Chem Phys. 1962;37:1003–7.
De Brabander C, Van Den Mooter G, Vervaet C, Remon JP. Characterization of ibuprofen as a nontradional plasticizer of ethyl cellulose. J Control Release. 2003;91:1678–85.
Breitzkreus J. Prediction of intestinal drug absorption properties by three-dimensional solubility parameters. Pharm Res. 1998;15:1370–5.
Blomqvist I, Westergren G, Sandberg A, Jonsson UE, Lundborg P. Pharmacokinetics and pharmacodynamics of controlled release metoprolol: a comparison with atenolol. Eur J Clin Pharmacol. 1988;33:S19–24.
Fang J, Semple HA, Song J. Determination of metoprolol, and its four metabolites in dog plasma. J Chromatogr B. 2004;809:9–14.
Lin SY, Yu HL. Microscopic Fourier transform infrared/differential scanning calorimetry systems used to study the different thermal behaviours of polymethacrylate copolymers of Eudragit RS, RL, E30D or E. J Appl Polym Sci. 2000;78:829–35.
Hancock BC, York P, Raymond CR. The use of solubility parameters in pharmaceutical dosage form design. Int J Pharm. 1997;148:1–21.
Greenhalgh D, Williams AC, Timmins P, York P. Solubility parameters as predictors of miscibility in solid dispersions. J Pharm Sci. 1999;88:1182–90.
Chokshi RJ, Sandhu HK, Iyer RM, Shah NH, Malick AW, Zia H. Characterization of physico-mechanical properties of indomethacin and polymers to assess their suitability for hot-melt extrusion process as a means to manufacture solid dispersion/solution. J Pharm Sci. 2005;94:2463–74.
Forster A, Hempenstall J, Tucker I, Rades T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int J Pharm. 2001;226:147–61.
Forster A, Hempenstall J, Rades T. Characterisation of glass solutions of poorly soluble drugs produced by melt extrusion with hydrophilic amorphous polymers. J Pharm Pharmacol. 2000;53:303–15.
Chokski RJ, Shah NH, Sandhu HK, Malick AW, Zia H. Stabilisation of low glass transition temperature indomethacin formulations: impact of polymer-type and its concentration. J Pharm Sci. 2007;97:2286–98.
Glaessl B, Siepmann F, Tucker I, Siepmann J, Rades T. Characterisation of quaternary polymethacryalate films containing tartaric acid, metoprolol free base or metoprolol tartrate. Eur J Pharm Biopharm. 2009;73:366–72.
Weyer L, Lo S-C. Handbook of vibrotional spectroscopy: spectra—structure correlations in the near-infrared. New York: Wiley; 2006.
Carli F, Capone G, Colombo I, Magarotto L, Motta A. Surface and transport properties of acrylic polymers influence drug release from porous matrices. Int J Pharm. 1984;21:317–29.
Glaessl B, Siepmann F, Tucker I, Rades T, Siepmann J. Deeper insight into the drug release machanism in Eudragit RL-based delivery systems. Int J Pharm. 2010;389:139–46.
Ragnarsson G, Sandberg A, Johansson MO, Lindstedt B, SJögren J. In vitro release characteristics of a membrane-coated pellet formulation—influence of drug solubility and particle size. Int J Pharm. 1992;79:223–32.
Ravishankar H, Patil P, Samel A, Petereit H-U, Lizio R. Controlled release by permeability alteration of cationic ammonio methacrylate copolymers using ionic interactions. Drug Dev Ind Pharm. 2006;32:709–18.
Wagner KG, McGinity JW. Influence of chloride ion exchange on the permeability and drug release of Eudragit RS 30 D films. J Control Release. 2002;82:385–97.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quinten, T., Andrews, G.P., De Beer, T. et al. Preparation and Evaluation of Sustained-Release Matrix Tablets Based on Metoprolol and an Acrylic Carrier Using Injection Moulding. AAPS PharmSciTech 13, 1197–1211 (2012). https://doi.org/10.1208/s12249-012-9848-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-012-9848-6