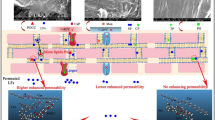
Abstract
Using tamsulosin (TAL) as a model drug, the aim of this study was to investigate and compare the percutaneous permeation behavior of two menthol derivatives, 2-isopropyl-5-methylcyclohexyl heptanoate (M-HEP) and 2-isopropyl-5-methylcyclohexyl decanoate (M-DEC). In vitro transdermal permeation study was carried out using porcine skin. The residual amount of enhancers in the skin after permeation experiment was determined by gas chromatographic (GC) method. The penetration depths of fluorescein were visualized by two-photon confocal laser scanning microscopy (2P-LSM) after the skin being treated with different enhancers. Furthermore, changes in the stretching frequency of functional group of ceramide were investigated by using attenuated total reflectance Fourier transform infrared (ATR-FTIR) technique. After M-HEP addition, the cumulative amount of TAL permeated in 8 h (Q 8) reached 20.57 ± 0.54 μg/cm2 and the depth of fluorescein was 40 μm; the CH2 of ceramide symmetric stretching frequency was 4 cm−1 blue shifted. However, M-DEC has an opposite effect on TAL permeation compared with that of M-HEP. TAL is a crucial factor affecting permeation procedure, and microenvironment of lipid region determines promotion capability of the enhancers.




Similar content being viewed by others
References
Zhao L, Li Y, Fang L, Ren C, Xu Y, He Z. Effect of O-acylmenthol and salt formation on the skin permeation of diclofenac acid. Drug Dev Ind Pharm. 2009;35:814–26.
Zhao L, Fang L, Xu Y, Zhao Y, He Z. Effect of O-acylmenthol on transdermal delivery of drugs with different lipophilicity. Int J Pharm. 2008;352:92–103.
Zhao L, Fang L, Xu Y, Liu S, He Z, Zhao Y. Transdermal delivery of penetrants with differing lipophilicities using O-acylmenthol derivatives as penetration enhancers. Eur J Pharm Biopharm. 2008;69:199–213.
Yu B, Kim KH, So PT, Blankschtein D, Langer R. Visualization of oleic acid-induced transdermal diffusion pathways using two-photon fluorescence microscopy. J Invest Dermatol. 2003;120:448–55.
Yu B, Dong CY, So PT, Blankschtein D, Langer R. In vitro visualization and quantification of oleic acid induced changes in transdermal transport using two-photon fluorescence microscopy. J Invest Dermatol. 2001;117:16–25.
Obata Y, Maruyama Y, Takavama K. The mode of promoting activity of O-ethylmenthol as a transdermal absorption enhancer. Pharm Res. 2006;23:392–400.
Narishetty ST, Panchagnula R. Effect of l-menthol and 1,8-cineole on phase behavior and molecular organization of SC lipids and skin permeation of zidovudine. J Control Release. 2005;102:59–70.
Takahashi K, Sakano H, Yoshida M, Numata N, Mizuno N. Characterization of the influence of polyol fatty acid esters on the permeation of diclofenac through rat skin. J Control Release. 2001;73:351–8.
Ibrahim SA, Li SK. Chemical enhancer solubility in human stratum corneum lipids and enhancer mechanism of action on stratum corneum lipid domain. Int J Pharm. 2010;383:89–98.
Fang L, Kobayashi Y, Numajiri S, Kobayashi D, Sugibayashi K, Morimoto Y. The enhancing effect of a triethanolamine-ethanol-isopropyl myristate mixed system on the skin permeation of acidic drugs. Biol Pharm Bull. 2002;25:1339–44. 17.
Hancock BC, York P, Rowe RC. The use of solubility parameters in pharmaceutical solid dosage form design. Int J Pharm. 1997;148:1–21.
Seto JE, Polat BE, VanVeller B, Lopez RF, Langer R, Blankschtein D. Fluorescent penetration enhancers for transdermal applications. J Control Release. 2012;158:85–92.
Krevelen V, Krevelen DW. Properties of Polymers. Amsterdam: Elsevier; 1990.
Dias M, Hadgraft J, Lane ME. Influence of membrane–solvent–solute interactions on solute permeation in skin. Int J Pharm. 2007;340:65–70.
Kuo TR, Wu CL, Hsu CT, Lo W, Chiang SJ, Lin SJ, Dong CY, Chen CC. Chemical enhancer induced changes in the mechanisms of transdermal delivery of zinc oxide nanoparticles. Biomaterials. 2009;30:3002–8.
Uitto OD, White HS. Electroosmotic pore transport in human skin. Pharm Res. 2003;20:646–52.
Alvarez-Román R, Naik A, Kalia YN, Fessi H, Guy RH. Visualization of skin penetration using confocal laser scanning microscopy. Eur J Pharm Biopharm. 2004;58:301–16.
Warner KS, Li SK, He N, Suhonen TM, Chantasart D, Bolikal D, Higuchi WI. Structure–activity relationship for chemical skin permeation enhancers: probing the chemical microenvironment of the site of action. J Pharm Sci. 2003;92:1305–22.
Warner KS, Li SK, Higuchi WI. Influences of alkyl group chain length and polar head group on chemical skin permeation enhancement. J Pharm Sci. 2001;90:1143–53.
Zhang CF, Yang ZL, Luo JB. Effects of cinnamene enhancers on transdermal delivery of ligustrazine hydrochloride. Eur J Pharm Biopharm. 2007;67:413–9.
Vaddi HK, Ho PC, Chan SY. Terpenes in propylene glycol as skin-penetration enhancers: permeation and partition of haloperidol, Fourier transform infrared spectroscopy, and differential scanning calorimetry. J Pharm Sci. 2002;91:1639–51.
Janůšová B, Zbytovská J, Lorenc P, Vavrysová H, Palát K, Hrabálek A, Vávrová K. Effect of ceramide acyl chain length on skin permeability and thermotropic phase behavior of model stratum corneum lipid membranes. Biochim Biophys Acta. 2011;1811:129–37.
He W, Guo X, Zhang M. Transdermal permeation enhancement of N-trimethyl chitosan for testosterone. Int J Pharm. 2008;356(1–2):82–7.
Acknowledgments
The program was supported by sub-subject of “new drug creating” of the Mega-Projects for Science Research for the “Eleventh Five-Year Plan” (No. 2009ZX09301-012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shang, L., Cun, D., Xi, H. et al. An Explanation for the Difference in the Percutaneous Penetration Behavior of Tamsulosin Induced by Two Different O-Acylmenthol Derivatives. AAPS PharmSciTech 15, 803–809 (2014). https://doi.org/10.1208/s12249-014-0105-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0105-z