Abstract
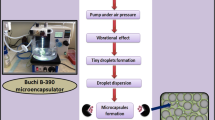
In previous studies, we developed and characterised multicompartmental microcapsules as a platform for the targeted oral delivery of lipophilic drugs in type 2 diabetes (T2D). We also designed a new microencapsulated formulation of probucol-sodium alginate (PB-SA), with good structural properties and excipient compatibility. The aim of this study was to examine the stability and pH-dependent targeted release of the microcapsules at various pH values and different temperatures. Microencapsulation was carried out using a Büchi-based microencapsulating system developed in our laboratory. Using SA polymer, two formulations were prepared: empty SA microcapsules (SA, control) and loaded SA microcapsules (PB-SA, test), at a constant ratio (1:30), respectively. Microcapsules were examined for drug content, zeta potential, size, morphology and swelling characteristics and PB release characteristics at pH 1.5, 3, 6 and 7.8. The production yield and microencapsulation efficiency were also determined. PB-SA microcapsules had 2.6 ± 0.25% PB content, and zeta potential of −66 ± 1.6%, suggesting good stability. They showed spherical and uniform morphology and significantly higher swelling at pH 7.8 at both 25 and 37°C (p < 0.05). The microcapsules showed multiphasic release properties at pH 7.8. The production yield and microencapsulation efficiency were high (85 ± 5 and 92 ± 2%, respectively). The PB-SA microcapsules exhibited distal gastrointestinal tract targeted delivery with a multiphasic release pattern and with good stability and uniformity. However, the release of PB from the microcapsules was not controlled, suggesting uneven distribution of the drug within the microcapsules.





Similar content being viewed by others
References
Barbeau WE, Bassaganya-Riera J, Hontecillas R. Putting the pieces of the puzzle together—a series of hypotheses on the etiology and pathogenesis of type 1 diabetes. Med Hypotheses. 2007;68(3):607–19.
Moore PA, Zgibor JC, Dasanayake AP. Diabetes: a growing epidemic of all ages. J Am Dent Assoc. 2003;134(Spec No):11S–5S.
Negrulj R, Mooranian A, Al-Salami H. Potentials and limitations of bile acids in type 2 diabetes: applications of microencapsulation as a novel oral delivery system. J Endocrinol Diabetes Mellitus. 2013;1:1–11.
Cani PD et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50(11):2374–83.
Goldfine AB, Fonseca V, Shoelson SE. Therapeutic approaches to target inflammation in type 2 diabetes. Clin Chem. 2011;57(2):162–7.
Wu R et al. Probucol ameliorates the development of nonalcoholic steatohepatitis in rats fed high-fat diets. Dig Dis Sci. 2013;58(1):163–71.
Yamashita S, Matsuzawa Y. Where are we with probucol: a new life for an old drug? Atherosclerosis. 2009;207(1):16–23.
Shimizu H et al. Probucol attenuated hyperglycemia in multiple low-dose streptozotocin-induced diabetic mice. Life Sci. 1991;49(18):1331–8.
Russell JC et al. Cardioprotective effect of probucol in the atherosclerosis-prone JCR:LA-cp rat. Eur J Pharmacol. 1998;350(2–3):203–10.
Zimetbaum P, Eder H, Frishman W. Probucol: pharmacology and clinical application. J Clin Pharmacol. 1990;30(1):3–9.
Vedantham K et al. Development of a probucol-releasing antithrombogenic drug eluting stent. J Biomed Mater Res B Appl Biomater. 2012;100(4):1068–77.
Nourooz-Zadeh J et al. Measurement of plasma probucol levels by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1994;654(1):55–60.
Ajun W et al. Preparation of aspirin and probucol in combination loaded chitosan nanoparticles and in vitro release study. Carbohydr Polym. 2009;75(4):566–74.
Mooranian A, Negrulj R, Martinez J, Mathavan S, Sciarretta J, Chen-Tan N, et al. Stability and release kinetics of an advanced gliclazide-cholic acid formulation: the use of artificial-cell microencapsulation in slow release targeted oral delivery of antidiabetics. J Pharm Innov, 2014 (in press).
Mooranian A, Negrulj R, Martinez J, Mathavan S, Sciarretta J, Chen-Tan N, Mukkur TK, Mikov M, Lalic-Popovic M, Stojančević M, Golocorbin-Kon S, Al-Salami H, A complex microencapsulated system: a platform for optimised oral delivery of antidiabetic drug-bile acid formulations. Pharmaceutical Development and Technology, 2014 (in press).
Pal D, Nayak AK. Novel tamarind seed polysaccharide-alginate mucoadhesive microspheres for oral gliclazide delivery: in vitro-in vivo evaluation. Drug Deliv. 2012;19(3):123–31.
Awasthi R, Kulkarni GT. Development of novel gastroretentive drug delivery system of gliclazide: hollow beads. Drug Dev Ind Pharm, 2013(0):1–11.
Mooranian A, et al. Stability and release kinetics of an advanced gliclazide-cholic acid formulation: the use of artificial-cell microencapsulation in slow release targeted oral delivery of antidiabetics. J Pharm Innov, 2014:1–8.
Mooranian A, et al. Novel artificial cell microencapsulation of a complex gliclazide-deoxycholic bile acid formulation: a characterisation study. Drug Des Dev Ther, 2014 (in press).
Mladenovska K et al. 5-ASA loaded chitosan–Ca–alginate microparticles: preparation and physicochemical characterization. Int J Pharm. 2007;345(1):59–69.
Abdelbary A, El-Gendy NA, Hosny A. Microencapsulation approach for orally extended delivery of glipizide: in vitro and in vivo evaluation. Indian J Pharm Sci. 2012;74(4):319–30.
Duro R et al. The adsorption of cellulose ethers in aqueous suspensions of pyrantel pamoate: effects on zeta potential and stability. Eur J Pharm Biopharm. 1998;45(2):181–8.
Xie HG et al. Effect of surface wettability and charge on protein adsorption onto implantable alginate chitosan alginate microcapsule surfaces. J Biomed Mater Res Part A. 2010;92(4):1357–65.
George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review. J Control Release. 2006;114(1):1–14.
Yang L et al. Physicochemical and biological characterization of monoketocholic acid, a novel permeability enhancer. Mol Pharm. 2009;6(2):448–56.
Mikov M et al. Pharmacology of bile acids and their derivatives: absorption promoters and therapeutic agents. Eur J Drug Metab Pharmacokinet. 2006;31(3):237–51.
Mikov M et al. Pharmacokinetics and hypoglycaemic effect of 3 alpha, 7 alpha-dihydroxy-12-oxo-5beta-cholanate (MKC) in diabetic rat. FEBS J. 2006;273:210.
Al-Salami H et al. Probiotic treatment proceeded by a single dose of bile acid and gliclazide exert the most hypoglycemic effect in type 1 diabetic rats. Med Hypothesis Res. 2008;4(2):93–101.
Mikov M et al. The influence of 3alpha, 7alpha-dihydroxy-12-keto-5beta-cholanate on gliclazide pharmacokinetics and glucose levels in a rat model of diabetes. Eur J Drug Metab Pharmacokinet. 2008;33(3):137–42.
Al-Salami H et al. Influence of the semisynthetic bile acid (MKC) on the ileal permeation of gliclazide in healthy and diabetic rats. Pharmacol Rep. 2008;60(4):532–41.
Al-Salami H et al. Probiotics decreased the bioavailability of the bile acid analog, monoketocholic acid, when coadministered with gliclazide, in healthy but not diabetic rats. Eur J Drug Metab Pharmacokinet. 2012;37(2):99–108.
Al-Salami H et al. Gliclazide reduces MKC intestinal transport in healthy but not diabetic rats. Eur J Drug Metab Pharmacokinet. 2009;34(1):43–50.
Al-Salami H et al. Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur J Drug Metab Pharmacokinet. 2008;33(2):101–6.
Al-Kassas RS, Al-Gohary OM, Al-Faadhel MM. Controlling of systemic absorption of gliclazide through incorporation into alginate beads. Int J Pharm. 2007;341(1–2):230–7.
Efentakis M, Buckton G. The effect of erosion and swelling on the dissolution of theophylline from low and high viscosity sodium alginate matrices. Pharm Dev Technol. 2002;7(1):69–77.
Silva CM et al. Insulin encapsulation in reinforced alginate microspheres prepared by internal gelation. Eur J Pharm Sci. 2006;29(2):148–59.
Andersen E, Karlaganis G, Sjovall J. Altered bile acid profiles in duodenal bile and urine in diabetic subjects. Eur J Clin Investig. 1988;18(2):166–72.
Cook MT et al. Microencapsulation of probiotics for gastrointestinal delivery. J Control Release. 2012;162(1):56–67.
Duboc H et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62(4):531–9.
Caesar R, Fak F, Backhed F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J Intern Med. 2010;268(4):320–8.
Cani PD et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–103.
Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73(2):121–36.
Narasimhan B, Langer R. Zero-order release of micro-and macromolecules from polymeric devices: the role of the burst effect. J Control Release. 1997;47(1):13–20.
Acknowledgments
The authors acknowledge the CHIRI at Curtin University and the Curtin-seeding grant for the support and also acknowledge the use of equipment, scientific and technical assistance of the Curtin University Electron Microscope Facility, which has been partially funded by the University, State and Commonwealth Governments.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mooranian, A., Negrulj, R., Al-Sallami, H.S. et al. Probucol Release from Novel Multicompartmental Microcapsules for the Oral Targeted Delivery in Type 2 Diabetes. AAPS PharmSciTech 16, 45–52 (2015). https://doi.org/10.1208/s12249-014-0205-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0205-9