Abstract
This study aims to prepare drotaverine hydrochloride superporous hydrogel hybrid systems (DSHH systems) to prolong its residence time in the stomach, provide extended release and reduce its frequency of administration. Drotaverine hydrochloride (DRH) is a spasmolytic drug that suffers from brief residence due to intestinal hypermotility during diarrheal episodes associated with gastrointestinal colics resulting in low bioavailability and repeated dosing. Eight DSHH systems were prepared using gas blowing technique. The prepared DSHH systems were evaluated regarding their morphology, incorporation efficiency, density, porosity, swelling ratio, viscoelastic property, erosion percentage and release kinetics. The FH8 formula containing equal proportion of chitosan (3%) /polyvinyl alcohol (3%) as strengthener and crosslinked with tripolyphosphate showed the highest incorporation efficiency (91.83 ± 1.33%), good swelling ratio (28.32 ± 3.15% after 24 h), optimum viscoelastic properties (60.19 ± 3.82 kPa) and sustained release profile (88.03 ± 2.15% after 24 h). A bioequivalence study was done to compare the bioavailability of the candidate formula versus Spasmocure®. Statistical analysis showed significant (P < 0.05) increase in bioavailability 2.7 folds with doubled Tmax (4 h) compared to the marketed product (2 h). These results declared that the superporous hydrogel hybrid systems could be a potential gastroretentive approach for the sustained delivery of drugs with short residence time with enhanced viscoelasticity.
Graphical abstract

Similar content being viewed by others
Introduction
Drotaverine hydrochloride (DRH) is a spasmolytic drug that suffers from short residence time; it is used for the relaxation of smooth muscle spasms and to relief the pain related to abdominal colics, spastic colon, biliary colics, ureteric colics, postoperative spasm and dysmenorrhea (1, 2). Oral administration of controlled release systems represents a very attractive approach for delivery of drugs with short residence time presenting a safe, convenient and economical way of drug delivery although there is a big challenge to localize such systems in case of intestinal hypermotility (3, 4).
For decreasing DRH dosing frequency, traditional sustained dosage forms are of little use, because of intestinal hypermotility associated with diarrheal episodes during bowel spasms could dislodge the dosage form from the intestine, thus reducing drug residence in the absorption window. Another approach is used to improve the drug’s efficacy by prolonging the drug gastric residence time through gastroretentive formulation. Gastroretentive dosage forms could extend the gastric residence time of drug and improve the bioavailability by increasing the duration of drug release through its absorption window and improving the solubility of drugs that are less soluble at higher pH environment (5, 6). Gastroretentive dosage forms can be broadly categorized into the following systems: magnetic, expandable, floating, bioadhesive, superporous hydrogel and high density systems (7).
Superporous hydrogel systems (SPH) were primarily designed as controlled delivery systems for retaining drugs in the stomach. These systems have a three-dimensional network of a hydrophilic polymer which absorbs a large quantity of water in a very short time because of the presence of interconnected microscopic pores. Gas blowing technique is mainly used to synthesize SPH systems by producing cellular structure within a polymer matrix (8, 9). Superporous hydrogel systems were classified into three generations, conventional superporous hydrogel system, superporous hydrogel composite system and superporous hydrogel hybrid system (10). Superporous hydrogel hybrid system (SHH) was developed to overcome the drawbacks associated with conventional superporous hydrogel system related to their poor elastic strength, as they were difficult to handle without breaking (11).
Conventional superporous hydrogel system was modified by Baek et al. 2001 (12) to form superporous hydrogel composite system by adding superdisintegrant which improved the mechanical properties and acted as the local point of physical entanglement of the polymer chains. However, they were still brittle and breakable. SHH system prepared by Omidian et al. 2007 (13) had high elastic and mechanical properties. Unlike composite system, a pre-crosslinked swelling additive was added. SHH system was prepared by adding a strengthener such as guar gum, gelatin, carboxymethyl cellulose, sodium alginate, chitosan, polyvinyl alcohol or gellan gum which could be crosslinked after superporous hydrogel preparation by postcrosslinker aqueous solution of glutaraldehyde, ferric chloride, calcium chloride, tripolyphosphate or sodium borate according to the type of strengthener used to create an interpenetrating network with high mechanical stability. Interpenetrating network (IPN) is a combination of two or more polymers in network form crosslinked with each other. Elastic swollen SHH system can resist various types of stresses including tension, compression, bending and twisting (14).
Drotaverine hydrochloride was formulated by Prakash et al. 2013 onto gastroretentive floating tablets (15) and onto gastroretentive floating alginate beads by Adel and Elkasabgy 2014 (16). Louis et al. 2020 formulated DRH into floating gastroretentive mini-tablets (17). The current study aims to formulate DRH into superporous hydrogel hybrid system as a viscoelastic gastroretentive approach to prolong its gastric residence time so as to extend its release through its absorption window, reduce the dosing frequency and enhance the compliance.
Materials and Methods
Materials
AlphaAmoun (Egypt) has kindly supplied Drotaverine hydrochloride (DRH) and the marketed product Spasmocure® tablets (60 mg). Acrylamide (AM), guar gum (GG), gelatin, pluronic f-127 (PF127), carboxymethylcellulose sodium salt (CMC) and polyvinyl alcohol (PVA) were procured from Sigma-Aldrich (USA). Acrylic acid (AA), N,N’-methylenebisacrylamide (BIS), sodium tripolyphosphate (TPP) and sodium borate were procured from Alfa Aesar (Germany). Ammonium persulfate (APS), N,N,N,N tetramethylethylenediamine (TEMED) and sodium alginate (ALG) were delivered from Fischer (UK). Glutaraldehyde (GL) was purchased from Acros (Belgium). Chitosan (CS) was delivered from Marine (India). N-Hexane was purchased from Tedia (USA). All other reagents and chemicals used were of analytical reagent grade.
Preparation of Superporous Hydrogel Hybrid Systems (DSHH systems)
Eight formulae of superporous hydrogel hybrid systems were prepared adopting gas blowing technique according to the method described by El-said et al. 2014 (18) and Sharma and Dhingra 2016 (19). Acrylamide (AM) solution (monomers) with crosslinker N,N’-methylenebisacrylamide (BIS) was poured into a glass beaker and shaken using a vortex shaker (Stuart SA7; Staffordshire, UK) for 10 s. Pluronic f-127 (PF127) (foaming stabilizer) and drotaverine hydrochloride (DRH) were added to the solution. Different types of hybrid agent (strengthener) were used, and the pH was adjusted to 5 using acetic acid. Then, ammonium persulfate (APS) and N,N,N,N tetramethylethylenediamine (TEMED) were added to the mixture as initiator and initiator catalyst, respectively, to induce the polymerization at room temperature. Sodium bicarbonate (foaming agent) was added to the mixture 30 s. after the addition of APS and TEMED.
The superporous hydrogel was formed and then removed from the beaker after 10 min. Then, it was dipped immediately in solution of post crosslinker (depending on the type of hybrid agents). Finally, the prepared DSHH systems were dried out overnight to a constant weight in the oven at 60 °C. The detailed composition of the prepared DSHH systems is shown in Table I.
Morphological Examination
FH1 as a representative sample of DSHH systems was photographed before and after being fully swollen in 0.1 N HCl (pH 1.2) after 12 h at ambient temperature. The porous structure and size of FH1 were also examined under the scanning electron microscope (SEM) (JSM-6390LV; JEOL, Tokyo, Japan). Dried sample was transversely cut by a scalpel. Then, it was fixed using double-sided adhesive tape on a brass stub and coated with a thin layer of gold under vacuum and made electrically conductive by (~ 150 Å) for 0.5 min. The micrographs were captured at an excitation voltage of 25 kV. (19, 20).
Incorporation Efficiency
A weighted amount of the DSHH systems corresponding to 60 mg of DRH was added to a beaker containing 100 ml of 0.1 N HCl and stirred for 24 h and assayed for DRH content using UV spectrophotometer at λmax = 353 nm (Shimadzu UV-1650 PC, Kyoto, Japan) (21). The experiment was repeated in triplicate using the following equation (22).
Apparent Density Measurement
The densities of the prepared DSHH systems were calculated using the following equation:
The volume of DSHH systems were determined by the solvent displacement method using hexane as the displacement fluid (23, 24).
Porosity Percentage
The porosity percentages of the prepared DSHH systems were measured using the solvent replacement method by immersing in absolute ethanol. The porosity percentages were calculated based on the following equation:
where W1 and W2 are the weight of the hydrogel before and after being immersed in absolute ethanol, respectively, \(\rho\) is the density of absolute ethanol and V1 is the initial volume of the hydrogel before being immersed in absolute ethanol (25, 26).
Swelling Study
Initially, the weight of completely dried DSHH systems was taken and then dipped in excess of 0.1 N HCl. The weight of DSHH systems at various time intervals after blotting excess water on the surface was determined. The swelling ratio is given by
where Ws is the weight in the swollen state and Wd is the weight in the dried state (27).
Viscoelastic Study
Viscoelastic properties of the swollen prepared DSHH systems were determined using Rheometer testing system (Physica MCR51, Anton Paar, Ostfildern, Germany). The cone and plate device was used with a cone diameter 15 mm. The prepared DSHH samples were swollen in 0.1 N HCl up to equilibrium (after 12 h). The swollen DSHH samples were mounted on the testing system and the excess of swollen hydrogel protruded beyond of the cone was trimmed (28). The amplitude sweep of the oscillatory test was used to determine the dynamic properties; the elastic modulus (storage modulus G') and viscous modulus (loss modulus G'') for the prepared DSHH systems. The average G' values of the prepared DSHH systems were determined (9, 29).
Erosion Determination
Erosion of the DSHH systems was determined according to the method described by Avachat and Kotwal 2007 (30). The samples were cut into uniform cylindrical shapes and then placed in a USP dissolution vessel containing 1000 ml of 0.1 N HCl (pH 1.2) at 37 ± 0.5 °C, and the apparatus was stirred at 100 rpm using a paddle. Samples were collected at time intervals 0.5, 1, 2, 3, 4, 6, 8, 12 and 24 h. The samples were dried in a vacuum oven at 40 °C until reaching to a constant weight. The degree of erosion E% was determined using the following equation:
where Wi and Wf are the weights of the initial and the same dried and partially eroded sample, respectively (31).
In Vitro Release Study of DRH from DSHH Systems
DRH release from DSHH systems was carried out for 24 h at 100 rpm in USP paddle dissolution apparatus (708-DS; Agilent, Waldbronn, Germany) at 37 ± 0.5 °C. Each DSHH system corresponding to 60 mg dose was put in 1 L 0.1 N HCl (corresponding to pH 1.2). The released DRH was spectrophotometrically assayed using (Shimadzu 1650 UV spectrophotometer, Kyoto, Japan) at the predetermined λmax using blank (0.1 N HCl). The cumulative released percentage of DRH was determined at each interval. The release profile of Spasmocure® tablets was used as a reference for comparison (17). Results are the average of 3 measurements (n = 3) (32).
Kinetic Study of the Release Data
The data of in vitro release profiles of the prepared DSHH systems were fitted in zero-order, first-order, Korsmeyer–Peppas and simplified Higuchi’s models, so as to determine the release kinetics and mechanism. The preference of a certain mechanism was based on the highest coefficient of determination (r2) (33, 34).
In Vivo Study of the Selected DSHH
The protocol of this study had the approval of Cairo University, Faculty of Pharmacy research ethics committee for clinical studies, Cairo, Egypt (PI 954). The adopted design is randomized open label parallel design consisting of healthy twelve male albino New Zealand rabbits (35, 36). Rabbits were assigned random numbers and divided into two dosing groups, each one consisted of six rabbits (37): group Ι was assigned for the candidate formula FH8 and group IΙ for Spasmocure® tablet.
An HPLC assay was used for the estimation of DRH in plasma with few modifications (38). The assay was carried out at room temperature using HPLC system (1260 Agilent, Waldbronn, Germany) consisting of Bondapak column (C18, 300 × 4.6 mm, 10μ) (Waters, Massachusetts, USA). The composition of the mobile phase is (55:45) acetate buffer pH 4.5: acetonitrile. The flow rate was set to 2 mL/min and a UV detector (Agilent 1260, Waldbronn, Germany) was used to analyze the eluent at λmax = 353 nm.
DRH stock solution serial dilution were prepared (50–2000 ng/ml). Hydrochlorothiazide (HTZ) was used as internal standard (100 μg/ml). The retention times of DRH and HTZ were 6.9 and 2.3 min, respectively. The accuracy and intra-/inter-batch precision of the assay were evaluated using control samples spiked at 3 levels for the calibration curve following replicate analysis (n = 9). The regression coefficient (r2) through the concentrations range of 50–2000 ng/ml was 0.9995. The relative bioavailability as well as the main pharmacokinetic data including Tmax, Cmax, AUC(0-∞) and AUC(0–24) was determined (39).
Results
Morphological Examination
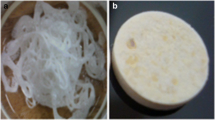
The morphology of the FH1 was optically examined in the dried and swollen states as illustrated in Fig. 1. It could be observed that the dried state of DSHH appeared to be rigid with smaller size, while swollen state exhibited larger size, and the porous structures of FH1 were examined under SEM (JSM- 6390LV; JEOL, Tokyo, Japan) as illustrated in Fig. 2.
It was previously found that the size of interconnected microscopic pores of superporous hydrogel systems was between 100 μm and 1000 μm (40, 41). Regarding DSHH systems, they possessed pore size of approximately 150 μm as illustrated in Fig. 2.
Incorporation Efficiency
The prepared superporous hydrogel hybrid systems exhibited high incorporation efficiency ranged from 86.85% ± 1.11% to 91.83% ± 1.33% as shown in Table II.
Apparent Density Measurement
The apparent densities of the prepared DSHH systems were found to vary between 0.84 ± 0.03 g/cm3 to 0.96 ± 0.01 g/cm3 as shown in Table II. The measured densities were related to the porosity of DSHH systems in terms of porosity percentage determined below. Also, the results of apparent densities of the DSHH systems are in accordance with the results obtained by Chavda et al. 2013 (42) and Bagadiya et al. 2011(43) which showed that the apparent densities were related to the porosity of the polymers of SHH systems.
Porosity Percentage
The porosity percentages of the prepared dried DSHH systems were found to be 45.19% ± 1.13% to 50.47% ± 1.37% as shown in Table II. The values of porosity percentage are linked to the apparent density and swelling ratio, where the apparent density of FH8 (0.96 ± 0.02 g/cm3) was significantly higher (p < 0.05) than other DSHH systems; this might be attributed to the smaller porosity percentage (45.19% ± 1.13%) of FH8 indicating the presence of less hollow pores occupying the same volume, thus leading to higher apparent density.
Swelling Studies
The swelling ratios of the DSHH systems ranged from 11.28% ± 0.96 to 18.5% ± 1.45 after 0.5 h and 28.53% ± 2.42 to 44.69% ± 3.45 after 12 h as shown in Fig. 3. It could be noted that the presence of the interpenetrating network (IPN) greatly influenced the swelling capability of the DSHH systems where their porous structure together with their IPN structure resulted in good swelling ratios.
Viscoelastic Study
Viscoelastic properties are obtained mainly by amplitude sweep of oscillatory tests which estimate small periodic deformations, structural breakdown or rearrangement of the SHH. Adequate elastic behavior of SHH depends mainly on their network structure. When the crosslinking density increased, the elastic properties increased at the expense of swelling. Good elastic behavior could withstand the pressure during gastric contraction and thereby prolonging the gastric retention time. Conventional superporous hydrogel systems with less elastic behavior could become fragmented after repetitive gastric contractions (28, 44).
The results of the storage modulus G’ and loss modulus G” of swollen DSHH systems are illustrated in Fig. 4. They showed gel-like characteristics (G’ > G”) (average G’ values 25.42 ± 2.54 kPa to 60.19 ± 3.82 kPa and average G” values 5.28 ± 0.05 kPa to 7.35 ± 1.22 kPa) which indicated that the elastic behavior is more dominant than the viscous behavior.
Erosion Determination
Viscoelastic property is a fundamental factor in determining the onset of erosion of DSHH systems. Swollen DSHH systems will erode after being highly hydrated since the intermolecular forces between polymer chains will not be able to withstand outside forces. Once DSHH systems eroded, they will break up into smaller particles, more surfaces will be exposed to swelling medium and hence more drug will be released. Since the erosion might affect the drug release, the erosion of the hydrated DSHH systems is important to be studied (45, 46).
DSHH systems have relatively low porosity percentage, low swelling ratio together with denser IPN structure compared to conventional SPH, which could lead to low erosion behavior. The erosion percentages for the prepared DSHH systems ranged from 0.17% ± 0.11% to 0.28% ± 0.22% after 0.5 h and 13.11% ± 2.17% to 18.35% ± 4.07% after 12 h as shown in Fig. 5.
In Vitro Release Study of DRH from DSHH Systems
It was noticed that DSHH systems could control DRH release where only 7.27% ± 1.91% to 15.48% ± 2.28% were released after 0.5 h and 80.12% ± 2.36 to 88.42% ± 2.68% were released after 12 h and it was also noticed that the percentage of DRH released from DSHH systems was lower than that from marketed product (Spasmocure® tablet) (90.18% ± 2.41% after 0.5 h) as shown in Fig. 6.
Kinetic Study of the Release Data
DRH release from the prepared DSHH systems best fitted to Korsmeyer–Peppas model as presented in Table III (47). Regarding DSHH systems, the “n” values were 0.514 to 0.723. These results indicated that DSHH system adopted non-Fickian model, suggesting that DRH release from SHH systems was controlled by degradation of the polymeric matrix as well as the drug diffusion.
In Vivo Study of the Selected DSHH
Plasma concentration–time curves of DRH following oral administration of DSHH FH8 and Spasmocure® tablet in rabbits are shown in Fig. 7. The first group of rabbits received the candidate formula FH8 in a dose equivalent to that of Spasmocure® tablet which is administered to the other group. The main pharmacokinetic data are presented in Table IV. Statistical analysis showed that the values of AUC(0-∞), Tmax and Cmax of DSHH formula (FH8) were significantly different (p˂0.05) compared to Spasmocure® tablet. Moreover, the Tmax was doubled and the bioavailability of the candidate formula (FH8) was 2.71 folds relative to that of Spasmocure® tablet.
Discussion
The SHH was developed as a gastroretentive approach with the aim of resolving the drawbacks of conventional SPH, through exhibiting enhanced viscoelastic behavior that allow them to withstand gastric contractions in order to maintain their swollen structure. Thus, they are expected to be retained in the stomach as they will not pass from the pyloric sphincter providing gastroretentive release behavior.
In the morphological examination, we could notice that there are many interconnected microscopic pores inside the prepared DSHH systems which might explain their ability of fast swelling and good swelling properties. These results were in accordance with Chen et al. 1998 (48) who reported that superporous hydrogel hybrid system had a porous structure that could lead to the formation of open capillary channels and fast swelling can be achieved via capillary wetting of interconnected pores i.e. water is rapidly absorbed by capillary attractive forces within the pores. Moreover, it was previously published by Qiu and Park 2003 (49) that swollen hydrogels were strong enough to withstand shear force and pressure generated in the stomach by gastric fluid so DSHH could potentially extend the gastric residence time of DRH. The formation of microscopic interconnected pores could be attributed to the addition of the hybrid agent which formed porous crosslinked structure through either chemical or physical crosslinking without compromising the mechanical and elastic properties of DSHH systems. The results were in agreement with the work done by Omidian et al. (10).
The system also exhibited high incorporation efficiency that might be credited to the integrated interpenetrating network (IPN) structure of DSHH systems which possibly increased the incorporation efficiency of DSHH by accommodating more drug in their crosslinked polymers network providing a reservoir of the drug in their structure matrix (50, 51).
The porosity percentage is the determining factor for the apparent density and swelling ratio. As systems having fewer hollow pores occupying the same volume, will possess higher apparent density and highest swelling ratio. The formula FH1 which exhibited the highest porosity percentage 50.47% ± 1.37% had the smallest density (0.84 ± 0.03) in contrast of FH8 which exhibited the lowest porosity percentage 45.19% ± 1.13% and the highest apparent density (0.96 ± 0.02).
Furthermore, the difference in porosity percentage could be reflected by the difference in the size and the number of the pores between the DSHH systems (52). The addition of the strengthener in the synthesis of DSHH systems didn’t induce the loss of pores but rather increased the viscosity of the reaction solution which resulted in retention of more bubbles in the polymer and kept the gel porosity.
However, a lot of strengtheners would induce bubbles to inflate and blow out so fewer pores were maintained in the polymer, which explained the diminished porosity of SHH systems relative to conventional and composite systems. These findings are in agreement with the work done by Yin et al. 2007 (25) and Patil-Vibhute and Hajare 2019 (53). Moreover, the porosity percentage of FH8 was significantly lower (p < 0.05) than other prepared DSHH systems; this might be due to the small size of pores of FH8 composed of CS and PVA, which developed intermolecular Van der Waals forces between the positively charged CS and the negatively charged hydroxylic group of PVA along with intermolecular hydrogen bonds which reduced the intermolecular spaces leading to decreased porosity percentage.
The use of CS and PVA as strengthener and the post-crosslinking with TPP as in FH8 exhibited significantly (p < 0.05) lower swelling ratio relative to the other DSHH systems which could be linked to its lower porosity percentage and higher apparent density. This might be due to the crosslinking between acrylate polymeric chains with the strengthener in a network form (54, 55).
As for the viscoelastic behavior of DSHH, the entanglement of acrylate chains with strengthener fibers developed the structural integrity of the hydrogel and decreased stress relaxation, which enhanced its ability to withstand pressure. The good elastic behavior of DSHH systems could indicate that they exhibited good mechanical strength. The greater elasticity of these formulations could be expected to enhance their retention in the stomach as previously described in Spence 1987 (56) and Ikeda and Nishinari 2001(57). The G’ value of FH8 formula (60.19 ± 3.82 kPa) was significantly higher (p < 0.05) than the other prepared DSHH systems, this might be attributed to the use of CS and PVA as a strengthener which exhibited Van der Waals interactions along with intermolecular hydrogen bonds and the post-crosslinking with TPP in the hybrid system (58, 59).
The erosion results are in accordance with the work done by Balamuralidhara et al. 2013 (32). FH8 showed the lowest percentage of erosion among DSHH systems with 0.17% ± 0.11% after 0.5 h and 13.11% ± 2.17% after 12 h; this might be attributed to exhibiting the lowest porosity percentage, swelling ratio together with highest apparent density which led to lower hydration rate and thereby slowed down its erosion.
The in-vitro release results could be attributed to the relatively low porosity, the denser interpenetrating network (IPN) structure and the relatively low erosion of DSHH systems. The low porous structure of DSHH system might be useful to allow lower diffusion of dissolution medium inside it, which might result in lower drug diffusion and hence a more delay in the drug release (44, 60, 61). Furthermore, the use of CS and PVA as strengthener and the post-crosslinking with TPP of FH8 led to greater sustainment of DRH release than all the other prepared DSHH systems own to their low erosion where 7.27% ± 1.91% were released after 0.5 h and 80.12% ± 2.36% were released after 12 h.
It was previously published that Korsmeyer–Peppas model has several simultaneous processes; diffusion of water into the DSHH, swelling of the DSHH as water enters formation of a gel, diffusion of drug and filler out of the DSHH and degradation of the polymer matrix. Regarding Spasmocure® 60 mg, the release data were best fitted to first order indicating that DRH release is affected by the concentration gradient (62, 63).
All these results might indicate that the candidate formula DSHH (FH8) composed of chitosan /polyvinyl alcohol crosslinked with tripolyphosphate displayed a gastroretentive release profile of DRH which resulted in extended drug absorption for 1 day along with higher bioavailability and gastroretentive behavior as reflected by the significantly delayed Tmax, (4 h compared to 2 h for the marketed product) higher bioavailability (271.83%).
Conclusion
A promising DRH gastroretentive system anticipated for once-daily dosing was successfully developed. The enhanced bioavailability along with prolonged release profile of DRH after oral administration of superporous hydrogel hybrid FH8 relative to marketed tablet could be related to the optimal swelling and viscoelastic properties of DSHH system that could prolong the gastric residence time providing sustained DRH absorption rate. Therefore, regarding FH8, a less frequent once daily dosing could be adopted. Consequently, the oral administration of DRH in the form of superporous hydrogel hybrid system (FH8) exhibiting enhanced viscoelastic behavior could be a favorable gastroretentive approach for a more convenient and effective delivery of drugs suffering from short gastric residence time.
References
Kumar D, Kumar A, Malik JK. Preformulation studies of drotaverine hydrochloride: an integral part of formulation design. European Journal of Biomedical and Pharmaceutical Sciences. 2019;6(13):304–7.
Mehmood Y, Mahmood RK, Akram W. Development and validation of UV-spectrophotometric methods for quantitative estimation of drotaverine HCl injection. Pharm Methods. 2017;8(1):31–5.
Patel P, Dand N, Somwanshi A, Kadam VJ, Hirlekar RS. Design and evaluation of a sustained release gastroretentive dosage form of captopril: a technical note. AAPS PharmSciTech. 2008;9(3):836–9.
Kushal M, Monali M, Durgavati M, Mittal P, Umesh S, Pragna S. Oral controlled release drug delivery system: an overview. Int Res J Pharm. 2013;4(3):70–6.
Kumar S, Jamil F, Rajput M, Sharma S. Gastro Retentive Drug Delivery System : Features and Facts. Int J Res Pharm Biomed Sci. 2012;3(1):125–36.
Fatima S, Usman S, Muhammad IN. Statistical evaluation of in-vitro dissolution profiles of different brands of simvastatin 20 mg tablets available in local market of Karachi. Int J Pharm Pharm Sci. 2013;5(3):622–6.
Surana AS, Kotecha RK. An overview on various approaches to oral controlled drug delivery system via gastroretension. Int J Pharm Sci Rev Res. 2010;2(2):68–72.
Gupta NV, Shivakumar HG. Development of a gastroretentive drug delivery system based on superporous hydrogel. Trop J Pharm Res. 2010;9(3):257–64.
Mayur C, Senthilkumaran K, Hemant G. Super porous hydrogels: a recent advancement in gastroretentive drug delivery system. Indones J Pharm. 2013;24(1):1–13.
Omidian H, Rocca JG, Park K. Advances in superporous hydrogels. J Control Release. 2005;102(1):3–12.
Sharma PK, Asthana GS, Asthana A. Hydrogel advancement: a new approach for gastroretentive drug delivery. Int J Pharm Clin Res. 2016;8(10):1418–22.
Baek N, Park K, Park JH, Bae YH. Control of the swelling rate of superporous hydrogels. J Bioact Compat Polym. 2001;16(1):47–57.
Omidian H, Park K, Rocca JG. recent developments in superporous hydrogels. J Pharm Pharmacol. 2007;59(3):317–27.
Nikunja B Pati, Swapna Velivela, V. M. and V. R. M. G. Gastroretentive superporous hydrogel tablets of dexlansoprazole. Int J Pharm Sci Res, 2016, 7 (11), 4678–4685.
Prakash, O.; Saraf, S.; Rahman, M.; Agnihotri, N.; Pathak, V. Formulation and evaluation of floating drotaverine hydrochloride tablets using factorial design. Res. J. Pharm. , Biol. Chem. Sci., 2013, 4 (4), 546–555.
Adel S, Elkasabgy NA. Design of innovated lipid-based floating beads loaded with an antispasmodic drug: in-vitro and in-vivo evaluation. J Liposome Res. 2014;24(2):136–49.
Louis, M.M.; Badawy, A.A.; Nessem, D.I.; Abd Elmalak, N.S. Drotaverine hydrochloride gastroretentive floating mini-tablets: Formulation, in-vitro and in-vivo evaluation, J. Drug Deliv. Sci. Technol. 57 (2020) 1–8.
El-said, I. A.; Aboelwafa, A. A.; Khalil, R. M.; ElGazayerly, O. N. Baclofen novel gastroretentive extended release gellan gum superporous hydrogel hybrid system: in vitro and in vivo evaluation. Drug Deliv., 2014, 23 (1), 101–112.
Sharma S, Dhingra G. Study of effect of initiators on synthesis of superporous hydrogels as gastroretentive device. Int J Pharm Pharm Res. 2016;8(1):106–15.
Kumar KA, Reddy AM, Babu PS. Preparation and characterization of swellable polymer based gastro-retentive zidovudine superporous hydrogel composite. Res J Pharm Sci. 2012;1(2):13–9.
Sharma PK, Asthana GS, Asthana A. Formulation development and evaluation of hydrogel based gastroretentive drug delivery system of antihypertensive drug. Int J Pharm Clin Res. 2016;8(10):1396–401.
Mukund JY, Kantilal BR, Sudhakar RN. Floating microspheres: a review. Brazilian J Pharm Sci. 2012;48(1):17–30.
Bhalla S, Nagpal M. Comparison of various generations of superporous hydrogels based on chitosan-acrylamide and in vitro drug release. ISRN Pharm. 2013;2013(1):1–8.
Pund AU, Shendge RS, Pote AK. Current approaches on gastroretentive drug delivery systems. Journal of Drug Delivery and Therapeutics. 2020;10(1):139–46.
Yin L, Fei L, Tang C, Yin C. Synthesis, characterization, mechanical properties and biocompatibility of interpenetrating polymer network–super- porous hydrogel containing sodium alginate. Polym Int. 2007;56:1563–71.
Lodge KB. The measurement of porosity for an individual taconite pellet. Powder Technol. 2010;204(1):167–72.
Kumar A, Reddy M, Manohara P, Babu S. A review on gastroretentive superporous hydrogels and its generations. J Chem Pharm Sci. 2012;5(2):78–81.
Salonen, L. Rheological and mechanical properties of hydrogels, tampere university of technology, 2014.
Law N, Doney B, Glover H, Qin Y, Aman ZM, Sercombe TB, Liew LJ, Dilley RJ, Doyle BJ. Characterisation of hyaluronic acid methylcellulose hydrogels for 3D bioprinting. J Mech Behav Biomed Mater. 2018;77:389–99.
Avachat A, Kotwal V. Design and evaluation of matrix-based controlled release tablets of diclofenac sodium and chondroitin sulphate. AAPS PharmSciTech. 2007;8(4):51–6.
Nwe N, Furuike T, Tamura H. The mechanical and biological properties of chitosan scaffolds for tissue regeneration templates are significantly enhanced by chitosan from gongronella butleri. Materials (Basel). 2009;2(2):374–98. https://doi.org/10.3390/ma2020374.
Balamuralidhara V, Pramod Kumar TM, Vishal Gupta N, Getyala A, Gangadharappa HV. Development of a novel biodegradable superporous hydrogel for gastroretentive application. Int J Polym Mater Polym Biomater. 2013;62(10):524–32.
Meka L, Kesavan B, Kalamata VN, Eaga CM, Bandari S, Vobalaboina V, Yamsani MR. Design and evaluation of polymeric coated minitablets as multiple unit gastroretentive floating drug delivery systems for furosemide. J Pharm Sci. 2009;98(6):2122–32.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23.
Narang M, Shah D, Akhtar H. Efficacy and safety of drotaverine hydrochloride in children with recurrent abdominal pain: a randomized placebo controlled trial. Indian Pediatr. 2013;2015(52):847–51.
Sonawane, R. O.; Patil, S. D. Fabrication and statistical optimization of starch-κ-carrageenan cross-linked hydrogel composite for extended release pellets of zaltoprofen. International Journal of Biological Macromolecules. Elsevier B.V 2018, pp 1–45.
Brian, E.; Rabe-Hesketh, S. Handbook of statistical analyses using stata, Fourth Edition; Chapman & Hall/CRC, 2006.
Sastry BS, Gananadhamu S, Rao GD. Development of Rp-Hplc method for estimation of drotaverine hydrochloride in pharmaceutical formulations. Int J Chem Sci. 2008;6(4):2055–61.
El-Kattan, A.; Varma, M. Preclinical pharmacokinetics: industrial perspective. In Mass Spectrometry Handbook; 2012; pp 107–118.
Mangla B, Rana V, Jain A. Gastroretentive drug delivery system : an overview. Asian Pacific J Heal Sci. 2017;4(4):141–54.
Chavda HV, Patel CN. Preparation and characterization of swellable polymer-based superporous hydrogel composite of poly (Acrylamide-Co-Acrylic Acid). Trends Biomater Artif Organs. 2010;24(2):83–9.
Chavda H, Modhia I, Mehta A, Patel R, Patel C. Development of bioadhesive chitosan superporous hydrogel composite particles based intestinal drug delivery system. Biomed Res Int. 2013;2013:1–11.
Bagadiya A, Kapadiya M, Mehta K. Superporous hydrogel : a promising tool for gastoretentive drug delivery system available online through. Int J Pharmacy & Technology. 2011;3(4):1556–71.
Mezger, T. G. The Rheology Handbook: for users of rotational and oscillation rheometers; Germany, 2002.
Chen J, Blevins WE, Park H, Park K. Gastric retention properties of superporous hydrogel composites. J Control Release. 2000;64(1–3):39–51.
Shwetha K, S. Development and evaluation of superporous hydrogels for metoprolol tartrate as a gastro retentive system. Indian J. Nov. Drug Deliv., 2012, 4 (2), 104–109.
Ritger, P. L.; Peppas, N. A. A simple equation for description of solute release ii. fickian and anomalous release from swellable devices. J. Control. Release, 1987, 5 (1), 37–42.
Chen J, Park H, Park K. Synthesis of superporous hydrogels: hydrogels with fast swelling and superabsorbent properties. J Biomed Mater Res. 1998;44(1):53–62.
Qiu Y, Park K. Superporous IPN Hydrogels having enhanced mechanical properties. AAPS PharmSciTech. 2003;4(4):E51.
Garg R, Gupta GD. Gastroretentive floating microspheres of silymarin: preparation and in vitro evaluation. Trop J Pharm Res. 2010;9(1):59–66.
Shariatinia Z, Jalali AM. Chitosan-based hydrogels : preparation, properties and applications. Int J Biol Macromol. 2018;115:194–220.
Juthi AZ, Wang B. Super porous hydrogel: a promising gastroretentive drug delivery system. World J Pharm Pharm Sci. 2017;6(5):129–59.
Patil-Vibhute PB, Hajare AA. Preparation and characterization of superporous hydrogel as gastroretentive drug delivery system for atenolol. Int J Pharm Sci Res. 2019;10(1):272–85.
Sampath TM, Gunathilake U, Ching YC, Chuah CH. Enhancement of curcumin bioavailability using nanocellulose reinforced chitosan hydrogel. Polymers (Basel). 2017;9(64):1–19.
Omidian H, Rocca JG, Park K. Elastic, superporous hydrogel hybrids of polyacrylamide and sodium alginate. Macromol Biosci. 2006;6(9):703–10.
Spence W. Slab pull and the seismotectonics of subducting lithosphere. Rev Geophys. 1987;25(1):55–69.
Ikeda S, Nishinari K. “Weak gel”-type rheological properties of aqueous dispersions of nonaggregated kappa-carrageenan helices. J Agric Food Chem. 2001;49:4436–41.
Gratieri T, Gelfuso GM, Rocha EM, Sarmento VH, de Freitas O, Lopez RFV. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur J Pharm Biopharm. 2010;75(2):186–93.
Ghosh K, Shu XZ, Mou R, Lombardi J, Prestwich GD, Rafailovich MH, Clark RAF. Rheological characterization of in situ cross-linkable hysluronan hydrogels. Biomacromol. 2005;6(5):2857–65.
Goganian AM, Hamishehkar H, Arsalani N, Khiabani HK. Microwave-promoted synthesis of smart superporous hydrogel for the development of gastroretentive drug delivery system. Adv Polym Technol. 2015;34(2):1–9.
Chandrakala R, Varun D, Narender M, Sunitha R. Exploitation Of second generation superporous hydrogel composites as matrix retaradants, in gel coaating of pregabalin formulation and in-vivo characterization. Int J Pharm Sci Res. 2018;9(12):5131–44.
Singhvi, G.; Singh, M. Review : in-vitro drug release characterization Models. Int. J. Pharm. Stud. Res., 2011, II (I), 77–84.
Singh, B.; Sharma, A.; Sharma, A. Design of antibiotic drug loaded carbopol- hydrogel for wound dressing applications abstract materials Used. Am. J. Drug Deliv. Ther., 2017, 4 (1:2), 1–9.
Acknowledgements
This work was supported by National organization for drug control and research (NODCAR), Giza, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Michael M. Farag and Mina M. Louis were responsible for data collection, interpretation and drafting of manuscript. Nevine S. Abd Elmalak, Alia A. Badawy and Demiana I. Nessem were involved in the design of the study and statistical analysis of data. Alia A. Badawy was responsible for critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farag, M.M., Louis, M.M., Badawy, A.A. et al. Drotaverine Hydrochloride Superporous Hydrogel Hybrid System: a Gastroretentive Approach for Sustained Drug Delivery and Enhanced Viscoelasticity. AAPS PharmSciTech 23, 124 (2022). https://doi.org/10.1208/s12249-022-02280-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02280-2