Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has resulted in rapid and regionally different approaches to breast cancer care.
Methods
In order to evaluate these changes, a COVID-19-specific registry was developed within the American Society of Breast Surgeons (ASBrS) Mastery that tracked whether decisions were usual or modified for COVID-19. Data on patient care entered into the COVID-19-specific registry and the ASBrS Mastery registry from 1 March 2020 to 15 March 2021 were reviewed.
Results
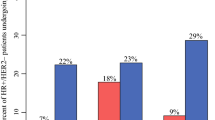
Overall, 177 surgeons entered demographic and treatment data on 2791 patients. Mean patient age was 62.7 years and 9.0% (252) were of African American race. Initial consultation occurred via telehealth in 6.2% (173) of patients and 1.4% (40) developed COVID-19. Mean invasive tumor size was 2.1 cm and 17.8% (411) were node-positive. In estrogen receptor-positive/human epidermal growth factor receptor 2-negative (ER+/HER2−) disease, neoadjuvant endocrine therapy (NET) was used as the usual approach in 6.9% (119) of patients and due to COVID-19 in an additional 31% (542) of patients. Patients were more likely to receive NET due to COVID-19 with increasing age and if they lived in the Northeast or Southeast (odds ratio [OR] 1.1, 2.3, and 1.7, respectively; p < 0.05). Genomic testing was performed on 51.5% (781) of estrogen-positive patients, of whom 20.7% (162) had testing on the core due to COVID-19. Patients were less likely to have core biopsy genomic testing due to COVID-19 if they were older (OR 0.89; p = 0.01) and more likely if they were node-positive (OR 4.0; p < 0.05). A change in surgical approach due to COVID-19 was reported for 5.4% (151) of patients.
Conclusion
The ASBrS COVID-19 registry provided a platform for monitoring treatment changes due to the pandemic, highlighting the increased use of NET.
Similar content being viewed by others
The two most widely recognized organizations that review and disseminate foundational information regarding human disease are the World Health Organization (WHO), originating in 1948, and the US Centers for Disease Control (CDC), which originated 2 years earlier in 1946.1,2 Each of these relatively ‘young’ multidisciplinary health organizations have spent the vast majority of 2020 and 2021 focusing on the global coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This pandemic has impacted every medical infrastructure, including disaster preparedness, supply chain capabilities, and health care worker resilience. As with prior large-scale ‘disasters’, which in modern history have been primarily meteorological events, disruptions in routine medical care often result in higher rates of urgent diseases such as trauma and infection as well as chronic disease progression and excess mortality.3 In February 2021, the WHO published that breast cancer has now overtaken lung cancer as the world’s most commonly diagnosed cancer, making effective and timely treatment for this disease even more imperative.4 Dr Sharpless, Director of the National Cancer Institute, with support from health care modeling experts, highlighted that an estimated excess of 10,000 deaths over the next 10 years from breast and colorectal cancer alone will likely occur due to the disruptions in screening and treatment caused by the COVID-19 pandemic.5
Early in the pandemic’s course, several large US-based medical societies, including the American Society of Breast Surgeons (ASBrS), the National Accreditation Program for Breast Centers (NAPBC), the National Comprehensive, Cancer Network (NCCN), the Commission on Cancer (CoC) and the American College of Radiology (ACR), collaborated to provide expert opinion regarding how best to prioritize patients diagnosed with breast cancer during this time period.6 These recommendations stratified patients based on patient disease acuity and the risk of disease progression versus the associated risks of an intervention based on hospital resources and patient and health care worker exposures. The recommendations provided by this group were very similar to those from international oncologic organizations and focused on prioritizing surgery for those completing neoadjuvant chemotherapy (NCT) for triple-negative and HER2-positive disease, as well as initiating neoadjuvant endocrine therapy (NET) for those with early-stage, hormone-positive malignancies, thus delaying the timing of surgery.7,8 The goal of these oncologic care guidelines was to help mitigate the potential impact of care disruptions while trying to prevent the spread of SARS-CoV-2.
In order to evaluate the impact of these expert opinion guidelines as well as the overall impact of the pandemic on the management of individuals with breast cancer in the US, the ASBrS leadership established a working group to develop a COVID-19 supplemental module to the established Mastery of Breast Surgery registry in March 2020. The ASBrS Mastery of Breast Surgery database was designed in 2006 as a web-based platform for physicians to document their breast procedures and surgeries as well as patient care outcomes, with the goal of permitting internal performance review and anonymized peer comparisons. The database includes de-identified, Health Insurance Portability and Accountability Act (HIPPA)-compliant information about patients, procedures, their risk stratification, and demographic data about the providers.9 The ASBrS COVID-19 Working Group hypothesized that during the pandemic, the number of patients treated with NET would increase in a regionally dependent manner due to the variability of ‘stay at home’ orders, the utilization of genomic testing on core biopsies would increase, and there would be both delays and/or alterations to patient’s breast surgeries. In this study, we provide the first 12 months of outcomes from this voluntary registry.
Methods
Between 13 March and 1 April 2020, the ASBrS COVID-19 Working Group developed a 22 question document for patient entry that included data on an individual patient’s treatment approach and whether this approach was part of usual care or due to the COVID-19 pandemic. All data were entered by the participating surgeons. The COVID-19 registry was a separate module within the established Mastery program to enable surgeons who did not routinely use the Mastery to track their patients impacted by the COVID-19 pandemic. Patients whose data were entered into the COVID-19-specific registry did not need to have corresponding additional data in the main Mastery modules. Surgeons who routinely used the Mastery could enter data into either the regular program and/or the COVID-19 registry and this would not duplicate patient information. The primary Mastery modules did not include data on whether care was influenced by COVID-19 and therefore patients without corresponding information in the COVID-19 registry were considered to be receiving usual care. If surgeons had not previously participated in the Mastery program they were asked to ensure compliance with the Mastery policies. The surgeons were asked to provide demographic information, including when their practice stopped and restarted mammographic screening, as well as if and when they stopped and started elective cancer surgery. The registry opened on 1 April 2020 and surgeons were asked to prospectively input cancer patients and to retrospectively provide data on those evaluated prior to March 2020 who were impacted by their hospitals’ changes due to the pandemic. Consecutive data entry is not required in this voluntary database. De-identified data from both the COVID-19 special registry and the standard Mastery database was obtained on 15 March 2021. Patients without a date of biopsy or diagnosis of invasive or in situ cancer were excluded, as were providers who entered fewer than four patients into either the COVID-19 or primary Mastery databases.
Descriptive data were used to describe the patient, tumor, and provider demographics using mean ± standard deviation for continuous variables and percentages for categorical variables. Multinomial regression models were used to determine the factors for primary and secondary outcomes, which included comparisons between patients for whom the intervention was usual care or due to the COVID-19 pandemic for (1) NET use, and (2) genomic testing use on the core biopsy versus the surgical specimen. Analyses were performed for invasive and ductal carcinoma in situ (DCIS) disease as separate cohorts, and statistical analyses were performed at the 0.05 significance level using R version 3.6.0 software (The R Foundation for Statistical Computing, Vienna, Austria). All data received from the Mastery database were de-identified. This study was considered exempt by the University of Wisconsin Institutional Review Board.
Results
Between 1 March 2020 and 15 March 2021, 177 surgeons entered data on 2791 unique patients (484 DCIS, 2307 invasive cancer) into the COVID-19 registry and on 9580 unique patients into the Mastery registry. Only 2980 (599 DCIS, 2381 invasive cancer) of the Mastery patients had biomarker information therefore the remaining 6600 patients were excluded. For patients whose data were entered into the COVID-19 registry, 43 were excluded due to the lack of biopsy date or the provider entered four or fewer patients in the registries. Thirty-three surgeons (18.6%) entered patient data into the Mastery registry only and did not use the COVID-19 registry. The majority (57.6%) of patients in the COVID-19 registry had a date of initial surgeon consult between 1 February 2020 and 30 April 2020. The provider demographic data are shown in Table 1. Regional surgeon distribution was Northeast, 36.7%; Midwest, 22%; Southeast, 16.9%; Southwest, 13%; and Northwest, 11.3%. The majority of surgeons entered between 10 and 99 patients (63.9%, 113 surgeons), were from urban/large urban areas (65%, 115 surgeons), and reported stopping mammographic screening at some point during the pandemic (94.9%). All surgeons reported having to stop some portion of their elective surgical schedule during the initial months of the pandemic, while a minority of surgeons had to stop all cancer surgery during the initial phases of the pandemic (24, 13.6%). These surgeons were primarily located in the Northeast (14, 58.3%).
Demographic and tumor information for those patients whose data were entered into the COVID-19 registry are shown in Table 2. Mean patient age was 62 years for those with DCIS and 63 years for those with invasive cancer, and 11.4% and 8.5% were of African American race, respectively. For the Mastery registry only, mean patient age was 62.5 years for those with DCIS and 64 years for those with invasive cancer, and 11% and 8.8% were of African American race, respectively. There were no missing data regarding patient age and race in either database. Within the COVID-19 registry, telehealth use of any type during the patient’s care was noted for 6.2% (173) of patients, and only 1.4% (40) developed COVID-19. The mean invasive tumor size was 2.1 cm, 17.8% (411) were node-positive and 10.2% (229) were triple-negative, 12.7% (286) were human epidermal growth factor receptor 2-positive (HER2+) and 77.1% (1731) were estrogen receptor-positive (ER+). The Mastery registry patients had 67% missing data on cancer size and 65% missing data on nodal status. These data are not shown due to the large number of missing variables.
Table 3 describes the treatment approaches for patients whose data were entered into the COVID-19 registry as well as those with available biomarker (70% missing) and treatment (36% missing) data in the Mastery registry. For those patients in the COVID-19 registry, mean time to primary surgery was 44.5 days. In ER+ invasive breast cancer, NET was used as a ‘usual’ approach in 5.4% (124) of patients in the COVID-19 registry, which was similar to 5.6% (286) of patients undergoing NET in the Mastery registry during this time period. NET was used due to COVID-19 in an additional 24.3% (560) of patients with invasive disease or 31.3% (542) of the ER+ patients. For patients with DCIS, NET was used due to COVID-19 in 30.8% (149) of patients. In multinomial regression (Table 4) with surgery first/usual practice as the reference, patients were more likely to receive NET due to COVID-19 and usual practice for both DCIS and invasive cancer with increasing age (odds ratio [OR] 1.09–1.4; p < 0.05), as well as if they lived in the Northeast or Southeast (OR 1.75–3.5; p < 0.05).
Genomic testing was performed in 781 patients, of whom 57.5% (449) received it on the surgical specimen (the majority as per usual practice, with just 12 patients classified as having genomic testing due to COVID-19). Genomic testing was performed on 332 core biopsy specimens—20.7% (162) due to COVID-19 and 21.8% (170) as per usual practice (Table 5). Of the 162 patients with genomic testing performed on their core specimen, 77% (125) proceeded on to NET and 10% (17) received NCT. Neoadjuvant therapy was more frequently used in patients with genomic testing on the core than in patients where genomic testing was performed on the surgical specimen. Genomic testing on the core biopsy occurred more frequently due to COVID-19 and also in patients who were node-positive than on the surgical specimen where the proportion of node-positive patients was lower. Patients were less likely to have genomic testing on the core due to COVID-19, as well as usual care, if they were older (OR 0.89, 0.86, respectively; p < 0.05), and more likely to have genomic testing if they were node-positive for both COVID-19 (25 patients) and usual care (49 patients) [OR 4.0, 8.9, respectively; p < 0.05].
Physicians were asked (1) would the patient return for another surgery (78% response rate), and (2) was the surgery changed or still pending due to COVID (100% response rate). The surgeons reported that 12.4% (271) of patients would return for another surgery at a later date, and the majority of these second surgeries were for a mastectomy, with or without reconstruction or for delayed reconstruction (41.3%, 112 patients). A change in surgical approach due to COVID-19 was reported for 5.4% (151) of patients, while 11.5% (322) of patients still had surgery pending at the time of this analysis. A wide variety of reasons were noted for an alteration in surgical treatment, with the most common result being reconstruction delayed with mastectomy performed without reconstruction for 24% (38) of patients, contralateral breast surgery postponed for 20% (32) of patients, and breast conservation performed when mastectomy with or without reconstruction was desired for 20% (32) of patients.
Discussion
This study analyzed practice patterns and management of patients diagnosed with breast cancer during the COVID-19 pandemic, specifically alterations in care due to the abrupt restrictions. Actions that were directly influenced by COVID-19 included 31% of ER+ patients receiving NET, 21% of eligible patients had genomic testing on the core biopsy specimen, and 12% of patients who underwent surgery will require additional surgeries. Additionally, almost all surgeons reported some or complete interruption to their elective surgical practice.
The COVID-19 pandemic has altered healthcare delivery and health outcomes worldwide and has resulted in decreased utilization of inpatient and outpatient services for non-COVID-19 medical conditions. The impact on cancer and non-cancer health outcomes may take years to fully realize.10 For those healthcare professionals who focus on breast diseases, there are several early hints at the changes caused by the COVID-19 pandemic.
In a study of six US academic centers during the first 3–4 months of the pandemic when all non-essential healthcare services were put on hold, screening and diagnostic mammography units saw fewer than 10% of their normal imaging patients.11 In another study of seven centers in North Carolina, it was noted that there was a 41% reduction in breast biopsy volumes in the first few months of the pandemic.12 In The Netherlands, Filipe et al. reported a drop to 40% of normal operative volumes for breast cancer patients in the first 3 months.13 In the first 6 months of the pandemic, in comparison with a similar time period in 2019, the number of referrals for a suspected breast cancer was 28% lower and the number of patients receiving their first treatment for breast cancer was 16% lower in England’s National Health Service Cancer Waiting Time database.14 In Italy, a single-institution study of patients treated from May to July 2020 versus the same time period in 2019 saw a 10% decrease in DCIS patients and an 11% increase in node-positive patients.15 A social media-based survey of 609 breast cancer patients that was distributed to breast cancer patient support groups found that 15 patients reported delays in surgery, 55 reported delays in reconstruction, and 26 reported delays in infusion/treatments.16
Anticipating several of these significant changes, including reduced mammographic screening, reduced access to operating rooms and infusion and radiation suites, and a need to transition as much care to an outpatient setting or telehealth, many national and international organizations, individually and in collaboration, created recommended guidelines for breast disease care.6,7,8,17 Leaders from the ASBrS, NAPBC, NCCN, CoC, and ACR formed the COVID-19 Pandemic Breast Cancer Consortium and wrote a prioritization paper using existing evidence-based data to help providers triage and treat breast cancer patients depending on the severity of the pandemic in their region, the type and stage of breast cancer, and the risk of COVID-19-related complications to the patient.6 Many estrogen and progesterone receptor-positive patients in Priority Group B were recommended to delay surgical treatment and initiate neoadjuvant hormonal therapy during the worst phases of the pandemic.
The finding of increasing use of NET and alterations in surgical management seen in our current study are in keeping with these guidelines and also some of the other recent reports in the literature.18 A comparative but consecutive patient entry study, the B-MaP-C, from 64 breast units in Britain, found that 59% (2246) of breast patients underwent COVID ‘altered’ care between March and May 2020. Twenty-five percent of 951 patients in the B-MaP-C received ‘bridging’ endocrine therapy, a similar increase to that seen in this ASBrS COVID-19 specific registry.19 In 2017, Chiba et al. reported that only 3% of cT2-4c patients older than 50 years of age were treated with NET, highlighting the low use of NET in patients in the US with hormone-sensitive breast cancer prior to the pandemic.20 In a June 2020 survey of US-based oncologic providers, 12% (13) of providers reported that they routinely used NET pre-COVID-19, while a reported 53% (114) of providers reported they would use short-term NET pre-surgically during COVID-19.21 Another provider survey by the European Breast Cancer Research Association reported that 68% (255) of physicians recommended endocrine therapy for luminal A tumors to enable surgery to be postponed during the pandemic.22 In randomized trials, NET has been shown to be safe and effective in increasing the rates of breast-conservation therapy.23,24,25,26
Both the current study and the British B-MaP-C study are likely the first large-scale patient-level evaluations on changes to breast cancer care during the initial months and year of the COVID-19 pandemic. Both studies showed that approximately 40–50% of patients were treated with standard or usual approaches; 41% of patients in the B-MaP-C study had standard surgery, chemotherapy, and/or radiation. In the ASBrS Mastery COVID-19 study, 46% (1288) of patients had surgery as part of usual care, while 65–69% (348) of those with HER2+ or triple-negative disease had recommended treatment with NCT. However, a proportion of patients had alterations in their surgical care due to resource management and the need to avoid prolonged hospitalizations. In the B-MaP-C study, 7.9% (299) of patients were not offered reconstruction; however, in the ASBrS COVID-19 Mastery study, 4.0% (112) of patients will return at a later date for mastectomy with reconstruction or reconstruction alone. These changes in care, which were necessary at the time, may result in longer-term psychologic consequences and increased health care costs due to multiple surgical interventions.
The use of genomic testing on the core biopsy samples of patients with estrogen-positive breast cancers was another anticipated change during the initial months of the COVID-19 pandemic. Genomic profiling has been shown to be effective in helping to determine the response to NET as well as the benefit of chemotherapy.27,28 In the ASBrS Mastery COVID-19 study, 10.7% (162) of patients underwent genomic testing on their core specimens to help guide therapy during the pandemic; 77% (125) went on to NET due to COVID-19, highlighting the key impact the testing had on this patient population, while another 10% (17) had NAC due to genomic testing results. As genomic testing becomes integrated into both standard treatment algorithms and clinical trials, evaluating novel neoadjuvant treatments post the pandemic, an increasing number of patients may undergo testing on the core biopsy sample in the future to help guide their clinical treatment. Further work regarding the correlation of genomic assay results on core biopsy samples versus surgical resection tissue is awaited.
There are several limitations to this special study. The ASBrS Mastery and COVID-19-specific registries are self-reported data entry systems by surgeons, which can be affected by response bias and lack of data verification. Additionally, consecutive patients were not required and may not be reflective of entire practices. Some data fields are optional, hence there were several datasets with missing entries. Lastly, the majority of patients in the COVID-19 registry were initially evaluated and treated in the first 4 months of the pandemic and may not be reflective of the entire picture of the pandemic that continued to evolve in 2020. However, most of the patient data in the dataset were entered by surgeons who have a significant portion of their practice treating breast cancer patients, the sample size was significant, and geographical variation was represented.
Conclusion
The impact of the COVID-19 pandemic on breast cancer management has been unprecedented, and interruption of screening, delays in treatment, alterations of standard management, and the requirement of follow-up surgery occurred as a result. Variations of these factors occurred temporally and geographically. In general, we have seen more personalized treatment plans with the increased use of genomic testing and NET. Likely, many of these treatment trends will persist after the pandemic has ended. A snapshot of care for breast cancer patients has been described in this study but the full impact is likely unrealized. Further research is needed to gain a more comprehensive understanding of the impacts of these treatment changes on long-term outcomes. It may be that we have moved towards higher value care as we strive to focus on the treatments that are most effective while balancing the potential exposure of patients to the SARS-CoV-2 virus.
References
Centers for Disease Control and Prevention. Our history—our story. https://www.cdc.gov/about/history/index.html. Accessed 14 Mar 2021.
World Health Organization. https://www.who.int/about/who-we-are/history. Accessed 14 Mar 2021.
Man RX-G, Lack DA, Wyatt CE, Murray V. The effect of natural disasters on cancer care: a systematic review. Lancet Oncol. 2018;19(9):e482–99.
World Health Organization. Breast cancer now most common form of cancer: WHO taking action. https://www.who.int/news/item/03-02-2021-breast-cancer-now-most-common-form-of-cancer-who-taking-action. Accessed 14 Mar 2021.
Sharpless NE. COVID-19 and cancer. Science. 2020;368(6497):1290.
Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020;181(3):487–97.
ESMO guidelines: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/breast-cancer-in-the-covid-19-era. Accessed 14 Mar 2021.
Burki TK. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020;5:629–30.
Clifford EJ, De Vol EB, Pockaj BA, Wilke LG, Boughey JC. Early results from a novel quality outcomes program: the American Society of Breast Surgeons’ Mastery of Breast Surgery. Ann Surg Oncol. 2010;17(Suppl 3):233–41.
Roy CM, Bollman EB, Carson LM, et al. Assessing the indirect effects of COVID-19 on healthcare delivery, utilization, and health outcomes: a scoping review. Eur J Public Health. 2021;31(3):634–40. https://doi.org/10.1093/eurpub/ckab047.
Norbash AM, Moore AV Jr, Recht MP, et al. Early stage radiology volume effects and considerations with the coronavirus disease 2019 (COVID-19) Pandemic: adaptations, risks, and lessons learned. J Am Coll Radiol. 2020;17(9):1086–95.
Nyante SJ, Benefield TS, Kuzmiak CM, et al. Population-level impact of coronavirus disease 2019 on breast cancer screening and diagnostic procedures. Cancer. 2021;127(12):2111–21. https://doi.org/10.1002/cncr.33460.
Filipe MD, van Deukeren D, Kip M, et al. Effect of the COVID-19 pandemic on surgical breast cancer care in the Netherlands: a multicenter retrospective cohort study. Clin Breast Cancer. 2020;20(6):454–61.
Gathani T, Glacyton G, MacInnes E, et al. The COVID-19 pandemic and impact on breast cancer diagnoses: what happened in England in the first half of 2020. Br J Cancer. 2021;124(4):710–2.
Toss A, Isca C, Venturelli M, et al. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open. 2021;6(2):100055. https://doi.org/10.1016/j.esmoop.2021.100055.
Papautsky EL, Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat. 2020;184(1):249–54.
Marron JM, Joffe S, Jagsi R, et al. Ethics and resource scarcity: ASCO recommendations for the oncology community during the COVID-19 pandemic. J Clin Oncol. 2020;38(19):2201–5.
Spring LM, Specht MC, Jimenez RB, et al. Case 22–2020: a 62-year-old woman with early breast cancer during the Covid-19 pandemic. N Engl J Med. 2020;383(3):262–72.
Dave RV, Kim B, Courtney A, et al. Breast cancer management pathways during the COVID-19 pandemic: outcomes from the UK “Alert Level 4” phase of the B-MaP-C study. Br J Cancer. 2021;124(11):1784–95. https://doi.org/10.1038/s41416-020-01234-4.
Chiba A, Hoskin TL, Heins CN, et al. Trends in neoadjuvant endocrine therapy use and impact on rates of breast conservation in hormone receptor-positive breast cancer: a national cancer data base study. Ann Surg Oncol. 2017;24(2):418–24.
Park KU, Gregory M, Bazan J, et al. Neoadjuvant endocrine therapy use in early stage breast cancer during the covid-19 pandemic. Breast Cancer Res Treat. 2021;188(1):249–58. https://doi.org/10.1007/s10549-021-06153-3.
Gasparri ML, Gentilini OD, Lueftner D, et al. Changes in breast cancer management during the Corona Virus Disease 19 pandemic: an international survey of the European Breast Cancer Research Association of Surgical Trialists (EUBREAST). Breast. 2020;52:110–5.
Eiermann W, Paepke S, Llombart-Cussac A, et al. Letrozole neo-adjuvant breast cancer study group preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized doubleblind multicenter study. Ann Oncol. 2001;12:1527–32.
Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the preoperative “Arimidex” compared to tamoxifen (PROACT) trial. Cancer. 2006;106(10):2095–103.
Ellis MJ, Suman VJ. Hoog J et al Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol. 2011;29(17):2342–9.
Dowsett M, Ellis MJ, Dixon JM, et al. Evidence-based guidelines for managing patients with primary ER+ HER2- breast cancer deferred from surgery due to the COVID-19 pandemic. NPJ Breast Cancer. 2020;6:21. https://doi.org/10.1038/s41523-020-0168-9.
Iwata H, Masuda N, Yamamoto Y, et al. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study. Breast Cancer Res Treat. 2019;173(1):123–33.
Bear HD, Wan W, Robidoux A, et al. Using the 21-gene assay from core needle biopsies to choose neoadjuvant therapy for breast cancer: a multicenter trial. J Surg Oncol. 2017;115(8):917–23.
Acknowledgments
A special thanks to ASBrS staff Mena Jalali and Margaret Scholsnagle for their expert assistance in providing the Mastery data, as well as a special thanks to the 177 surgeons who entered data on their breast cancer patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Lee G. Wilke: Founder/minority stock owner, Elucent Medical; institutional support for clinical trial/Perimeter Medical, unrelated to the current manuscript. Judy C. Boughey: Funding from Eli Lilly for a clinical trial, paid to the institution and unrelated to the current manuscript. Toan Thien Nguyen, Qiuyu Yang, Bret M. Hanlon, Kathryn A. Wagner, Pamela Strickland, Eric Brown, and Jill R. Dietz have no disclosures to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wilke, L.G., Nguyen, T.T., Yang, Q. et al. Analysis of the Impact of the COVID-19 Pandemic on the Multidisciplinary Management of Breast Cancer: Review from the American Society of Breast Surgeons COVID-19 and Mastery Registries. Ann Surg Oncol 28, 5535–5543 (2021). https://doi.org/10.1245/s10434-021-10639-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10639-1