Abstract
Background
Recently, there has been an increase in the availability of targeted molecular therapies for cancer treatment. The application of these approaches to esophageal cancer, however, has been hampered by the relative lack of appropriate models for preclinical testing. Patient-derived tumor xenograft (PDTX) models are gaining popularity for studying many cancers. Unfortunately, it has proven difficult to generate xenografts from esophageal cancer using these models. The purpose of this study was to improve the engraftment efficiency of esophageal PDTXs.
Methods
Fresh pieces of esophageal tumors obtained from endoscopic biopsies or resected specimens were collected from 23 patients. The tumors were then coated in Matrigel and transplanted in immunocompromised mice subcutaneously (n = 6) and/or using a novel implantation technique whereby the tumor is placed in a dorsal intramuscular pocket (n = 18). They are then monitored for engraftment.
Results
With the novel intramuscular technique, successful engraftment was achieved for all 18 patient tumors. Among these PDTXs, 13 recapitulated the original patient tumors with respect to degree of differentiation, molecular and genetic profiles, and chemotherapeutic response. Lymphomatous transformation was observed in the other five PDTXs. Successful engraftment was achieved for only one of six patient tumors using the classic subcutaneous approach.
Discussion
We achieved a much higher engraftment rate of PDTXs using our novel intramuscular transplant technique than has been reported in other published studies. It is hoped that this advancement will help expedite the development and testing of new therapies for esophageal cancer.


Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–6.
SEER Cancer Statistics Factsheets: Esophageal Cancer. Bethesda, MD: National Cancer Institute. Available: http://seer.cancer.gov/statfacts/html/esoph.html. Accessed 5 Nov 2014.
Tentler JJ, Tan AC, Weekes CD, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338–50.
Boonstra JJ, Tilanus HW, Dinjens WNM. Translational research on esophageal adenocarcinoma: from cell line to clinic. Dis Esophagus. DOI: 10.1111/dote.12095.
Dodbiba L, Teichman J, Fleet A, et al. Primary esophageal and gastro-esophageal junction cancer xenograft models: clinicopathological features and engraftment. Lab Invest. 2013;93:397–407.
Rumpel CA, Powell SM, Moskaluk CA. Mapping of genetic deletions on the long arm of chromosome 4 in human esophageal adenocarcinomas. Am J Pathol. 1999;154:1329–34.
Boonstra JJ, van Marion R, Douben HJ, et al. Mapping of homozygous deletions in verified esophageal adenocarcinoma cell lines and xenografts. Genes Chromosomes Cancer. 2012;51:272–82.
De Both NJ, Wijnhoven BPL, Sleddens HFBM, Tilanus HW, Dinjens WNM. Establishment of cell lines from adenocarcinomas of the esophagus and gastric cardia growing in vivo and in vitro. Virchows Arch. 2001;438:451–6.
El-Rifai W, Harper JC, Cummings OW, Hyytinen ER, Frierson HF Jr, Knuutila S, Powell SM. Consistent genetic alterations in xenografts of proximal stomach and gastro-esophageal junction adenocarcinomas. Cancer Res. 1998;58:34–37.
Zhang J, Jiang D, Li X, et al. Establishment and characterization of esophageal squamous cell carcinoma patient-derived xenograft mouse models for preclinical drug discovery. Lab Invest. 2014; 94:917–26.
Wang Z, Da Silva TG, Jin K, et al. Notch signaling drives stemness and tumorigenicity of esophageal adenocarcinoma. Cancer Res. 2014;74:6364–74.
Hidalgo M, Amant F, Biankin AV, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013.
Taniere P, Borghi-Scoazec G, Saurin JC, et al. Cytokeratin expression in adenocarcinomas of the esophagogastric junction: a comparative study of adenocarcinomas of the distal esophagus and of the proximal stomach. Am J Surg Pathol. 2002;26:1213–21.
Clemons NJ, Do H, Fennell C, Deb S, Fellowes A, Dobrovic A, Phillips WA. Characterization of a novel tumorigenic esophageal adenocarcinoma cell line: OANC1. Dig Dis Sci. 2014;59:78–88.
Lieber J, Eicher C, Wenz J, Kirchner B, Warmann SW, Fuchs J, Armeanu-Ebinger S. The BH3 mimetic ABT-737 increases treatment efficiency of paclitaxel against hepatoblastoma. BMC Cancer. 2011;11:362.
Wunderlich M, Mizukawa B, Chou F, Sexton C, Shrestha M, Saunthararajah Y, Mulloy JC. AML cells are differentially sensitive to chemotherapy treatment in a human xenograft model. Blood. 2013;121:e90–7.
Johnsson A, Olsson C, Nygren O, Nilsson M, Seiving B, Cavallin-Stahl E. Pharmacokinetics and tissue distribution of cisplatin in nude mice: platinum levels and cisplatin-DNA adducts. Cancer Chemother Pharmacol. 1995;37:23–31.
Desgranges C, Razaka G, De Clercq E, Herdewijn P, Balzarini J, Drouillet F, Bricaud H. Effect of (E)-5-(2-bromovinyl)uracil on the catabolism and antitumor activity of 5-fluorouracil in rats and leukemic mice. Cancer Res. 1986;46:1094–101.
Israely T, Dafni H, Granot D, Nevo N, Tsafriri A, Neeman M. Vascular remodeling and angiogenesis in ectopic ovarian transplants: a crucial role of pericytes and vascular smooth muscle cells in maintenance of ovarian grafts. Biol Reprod. 2003;68:2055–64.
Braunwald E. Regulation of the circulation. N Engl J Med. 1974;290:1420–5.
John T, Yanagawa N, Kohler D, et al. Characterization of lymphomas developing in immunodeficient mice implanted with primary human non-small cell lung cancer. J Thorac Oncol. 2012;7:1101–8.
Chen K, Ahmed S, Adeyi O, Dick JE, Ghanekar A. Human solid tumor xenografts in immunodeficient mice are vulnerable to lymphomagenesis associated with Epstein-Barr virus. PLoS One. 2012;7:e39294.
Vockerodt M, Yap LF, Shannon-Lowe C, Curley H, Wei W, Vrzalikova K, Murray PG. The Epstein-Barr virus and the pathogenesis of lymphoma. J Pathol. DOI: 10.1002/path.4459.
Acknowledgment
This work was supported by a NHMRC of Australia Centres for Research Excellence Grant (1040947). MR received the Thornell-Shore Memorial scholarship from the Royal Australasian College of Surgeons (RACS) and the Sir Thomas Naghten Fitzgerald scholarship from The University of Melbourne. DL received an NHMRC Australian Postgraduate Research award and Foundation for a Surgery Scholarship from the RACS. JS is supported by the Victorian Cancer Agency/Snowdome Foundation “Eva & Les Erdi Fellowship.” Some tissue samples used in this Project were provided by the Victorian Cancer Biobank with appropriate ethics approval. The Victorian Cancer Biobank is supported by the Victorian Government, Australia.
Disclosure
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nicholas J. Clemons and Wayne A. Phillips are co-senior authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10434_2015_4425_MOESM1_ESM.tif
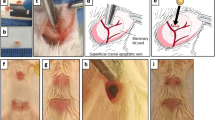
Supplementary Fig. 1 Intramuscular (IM) implantation technique and PDTX formation. a After placement of a stay suture, a small IM pocket is created on the dorsum of the mouse that is just large enough to accommodate the tumor piece. b After implantation, the IM pocket is sutured closed with the implant evident immediately below the outer muscle fibers. c Gross view of an IM PDTX at the time of harvest (TIFF 2961 kb)
10434_2015_4425_MOESM2_ESM.tif
Supplementary Fig. 2 Identification and characterization of lymphomagenic transformation within a subset of established PDTXs. Immunohistochemistry using pan-cytokeratin AE1/AE3 (1:100) (NCL-L-AE1/AE3; Novocastra/Leica Microsystems, Newcastle, UK), CD20 (1:200) (M7055, DakoCytomation; Dako, Glostrup, Denmark), and CD45 (1:200) (M0701, DakoCytomation) antibodies on the original patient tumor and the corresponding PDTX. The PDTX of patient 9 (a) is negative for the pan-cytokeratin marker AE1/AE3 but positive for the common leukocyte antigen CD45 and the B-lymphocyte restricted antigen CD20, indicating that it is predominantly composed of human B cells. In comparison, the PDTX of patient 16 (b) exhibits a combination of epithelial cells (nests of AE1/AE3-positive cells) and lymphocytes. c Further analysis via flow cytometry was performed as previously described (Craig FE, Foon KA. Blood 2008;222:3941–67) of single cells derived from PDTX 14 and confirmed that the lymphoid cells were CD19+ B cells (left plot). The abnormal B-cell population was then gated and is depicted on the FSC vs. SSC plot in blue (right plot), indicating that these cells are also large. d Right In situ hybridization for the Epstein Barr virus (EBV) (blue staining) was performed on sections of formalin-fixed paraffin-embedded PDTX using the INFORM EBER Probe and ISH iVIEW Blue Detection Kit (Ventana/Roche, Tucson, AZ, USA) as per the manufacturer’s protocol and counterstained with eosin (pink staining). Left EBV-positive nasopharyngeal carcinoma (positive control). Right Section from PDTX 9 (TIFF 3402 kb)
Rights and permissions
About this article
Cite this article
Read, M., Liu, D., Duong, C.P. et al. Intramuscular Transplantation Improves Engraftment Rates for Esophageal Patient-Derived Tumor Xenografts. Ann Surg Oncol 23, 305–311 (2016). https://doi.org/10.1245/s10434-015-4425-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4425-3