Manuscript accepted on :07-03-2023
Published online on: 20-03-2023
Plagiarism Check: Yes
Reviewed by: Dr. Abeer Gatea
Second Review by: Dr. Dewi Syahidah
Final Approval by: Dr. Patorn Promchai
Abduladeem G.M. Al-Selwi1,2,4* and Amina Barkat1,3
and Amina Barkat1,3
1Department: of Clinical epidemiology and medico-surgical sciences, Faculty of Medicine and Pharmacy, University Mohammed V, Rabat, Morocco
2Medical Research Laboratory, Children's Hospital, Ibn Sina University Hospital, Rabat, Morocco
3National Reference Center for Neonatology and Nutrition. Rabat Children's Hospital Ibn Sina, Rabat, Morocco
4Taiz University in Yemen
Corresponding Author E-mail: abdualadeem12@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2607
Abstract
Bacterial resistance to antibiotics has a very important role because it constitutes a threat to human health, especially immunocompromised people and children, this phenomenon can lead to difficulty or even the impossibility of treating certain infections. A meta-analysis from studies in Morocco on bacteria resistant to antibiotics over the last nine years and interest of bacterial: S. pneumoniae, N. meningitidis, H. influenzae and S. aureus, also the evolution their resistance. Total 654 articles in databases (206, 162, 134, and 152 articles found in: Elsevier, PubMed, Google Scholar, and other engines, respectively). For the bacteria in which we were interested, the prevalence of resistance increases with the years. Indeed, S. pneumoniae, N. meningitidis and H. influenzae, prevalence in 2012, 2016 and 2018 was respectively around (13%, 9.7%, 5.4%), (48%, 24%, 8%) and (29%, 33%, 8%). The evolution of the resistance of S. pneumoniae, was impacted by the introduction of the vaccine, indeed, the rate of its resistance to the antibiotic erythromycin before vaccination was 76% but after the introduction of the vaccine it decreased to 61%, while the incidence of pneumonia was 17.7%, and after vaccination it decreased to 10.2%. Also, the resistance of S. pneumoniae to penicillin G increased from 2.7% in 2011 to 100% in 2020. For N. meningitidis, resistance to penicillin G increased from 11.1% to 24% between 2012 and 2019. About of H. Influenzae for Bactrim, fluoroquinolones and tetracycline (16%, 4.8%, 2.5%), S.aureus resistance increases significantly. From 2016 to 2018, the resistance of S. aureus (Penicillin G 92%, ciprofloxacin 16.5%, erythromycin 14.6%).
Keywords
Antibiotics; Bacteria resistance; Haemophilus influenzae; Neisseria meningitidis; Streptococcus pneumoniae; Staphylococcus aureus
Download this article as:| Copy the following to cite this article: Al-Selwi A. G. M, Barkat A. Antibiotic resistance of Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae and Staphylococcus aureus in Morocco, national data: Meta- analysis. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Al-Selwi A. G. M, Barkat A. Antibiotic resistance of Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae and Staphylococcus aureus in Morocco, national data: Meta- analysis. Biomed Pharmacol J 2023;16(1).Available from: https://bit.ly/3yQn1fH |
Introduction
Antibiotic resistance is nowadays one of the serious problems in the field of global health. It is reaching significant proportions in all regions of the globe. Each day, new resistance mechanisms appear and spread around the world, compromising ability to treat the most common infectious diseases 1. Antibiotic resistance is reaching high levels worldwide, including Morocco and other neighboring African countries 2, with some bacteria becoming alarmingly multi-resistant to antibiotics 3. A bacterium is said to be resistant when it escapes the action of the supposedly active antibiotic prescribed to the patient, which is manifested by a relative or absolute clinical failure of the antibiotic therapy. In the majority of infections, a clinical failure results in the absence of improvement (fever, general condition) after approximately 72 hours of treatment and the prescription of a second antibiotic.4,5
There are several types of bacterial resistance, for example: natural bacterial resistance, acquired bacterial resistance, resistance by chromosomal mutation, resistance by gene acquisition, cross-resistance, co-resistance and selection 6. Two major reasons influencing this development are the widespread use of antibiotic therapy and the epidemic spread of resistant bacteria. However, other bacterial species long spared by this phenomenon and responsible for community infections (Streptococcus pneumoniae (S. pneumoniae), Neisseria meningitidis (N. meningitidis), Haemophilus influenzae (H. influenzae) and Staphylococcus aureus (S. aureus)) have in turn evolved in the direction of resistance 7.
S. pneumoniae is a species of bacteria of the genus Streptococcus, it is an important pathogen. It is responsible for many infections: for example, it has increased mortality during the Spanish flu pandemic. Its original name was diplodocus pneumoniae in 1926, it was named Streptococcus pneumoniae in 1974 due to its chain-like growth in liquid media. Because of its involvement as a pathogen in pneumonia, it has long been referred to simply as pneumococcus. Pneumococcal infections are particularly dangerous and very often require hospitalization, with mortality rates ranging from 8 to 15%.8. S. pneumoniae, a major cause of community acquired invasive infection for newborns and children. Invasive pneumococcal infections, including meningitis, remain worrisome with a mortality rate of over 8% and a significant risk of complications. Of more than 90 serotypes, only a limited number are responsible for pneumococcal infections. The incidence of serotype may vary depending on the age of the patient, geographic region, and time of surveillance. The advent and introduction of pneumococcal conjugate vaccines has resulted in significant progress in the prevention of pneumococcal 9.
N. meningitidis, known as meningococcus, is a gram-negative diplococcal bacterium known for its role in meningitis. Meningococci are germs found only in humans, in the nasopharynx, where they can cause mild nasopharyngitis or an asymptomatic carrier state. One can remain a carrier for several months or even years. In a normal population, 5-10% of carriers are found, but this rate can reach 50-75% in certain dense communities (barracks, boarding schools). Out of approximately 400 carriers, only one person becomes a victim of a serious meningococcal infection, this is most often presented as acute purulent meningitis. The meningococcus could move from the nasopharynx to the meninges by following the path of the olfactory nerves. However, the meningococcus reaches central nervous system (CNS) through bloodstream. Indeed, meningococcal disease almost always present at the beginning of meningitis. This sepsis may be asymptomatic, or it may add to the meningeal syndrome a purpuric eruption (found, depending on the epidemic, in 10 to 50% of cases of meningitis), or in 5 to 10% of cases, it may present as purpura fulminans, rapidly fatal, even before meningitis develops. These superacute forms are referred to as Waterhouse-Friderichsen syndrome. In these cases, the endotoxin, by contracting the intrahepatic veins, causes the accumulation of blood upstream of these veins, resulting in capillary hemorrhages (in the adrenals among others), intravascular thrombosis and circulatory collapse 10,11.
H. influenzae, called Pfeiffer’s bacillus, is a bacterium of the family: Pasteurellacae and class: Gammaproteobacteria. The cells are coccobacilli or small immobile Gram-negative rods. Richard Pfeiffer (1858-1945) was the first to describe them in 1892 from the influenza pandemic of 1889-1892. For a long time, he was believed to be responsible for influenza, until 1931, when Richard Shope isolated a virus from filtrates of pig lung shreds during a swine influenza similar to the human one 12,13. H. influenzae is a species sensitive to many families of antibiotics, and has not been spared the evolution of antibiotic resistance. The level of acquired resistance for aminpenicillins, tetracycline and trimethoprim places H. influenzae in the species inconsistently susceptible to these antibiotics, this species is naturally resistant to lincosamides and is classified as resistant to macrolides with a 16 atom cycle 14.
S. aureus is most pathogenic species of the genus Staphylococcus. It is responsible for localized suppurative infections, food poisoning, and in some extreme cases, infections that could be fatal (immunocompromised patients, cardiac prostheses). S.aureus presents itself as a clustered shell (Grape clusters), Gram positive and catalase positive. Its name derives from the golden tint that its carotenoid concentration provides it 15. The S. aureus species is commensal to humans (it is present in 15 – 30% of individuals known as healthy carriers in whom it has an ecological protection role), is an opportunistic pathogen in certain locations, under certain circumstances. S.aureus a good resistance to natural purification mechanisms (oxidation, desiccation, which explains its direct but also indirect transmission). S.aureus is a germ that has developed a remarkable level of resistance against multiple antibiotics, complicating treatment. Historically, S. aureus resistance appeared within two years after introduction penicillin 3. Controlling infections caused by antibiotic-resistant bacteria in humans requires regular monitoring of bacterial resistance and control of antibiotic use 16.
The frequency of the appearance of resistance and multi-resistance is most often conditioned by an increased use of antibiotics. The pressure of these molecules on the bacterial flora seems to be at the origin of the emergence of bacterial resistance. Therefore, on the recommendation of the World Health Organization (WHO), structures for monitoring antibiotic resistance and committees on the proper use of these molecules have been set up in most countries of the world. The objective of these structures is to periodically take stock of bacterial resistance in order to better adapt antibiotic therapy 17,18.
This paper aim is to make a meta-analysis from the studies done in Morocco on antibiotic resistant bacteria during the last nine years (2011-2020). We were interested in the following bacteria: (S. pneumoniae, N. meningitidis, H. influenzae and S. aureus) and the evolution this resistance.
Materials and methods
A thorough meta-analysis of past studies on the topic of bacterial resistance to antibiotics was carried out using national data in Morocco, for relatively common pathogens occasionally responsible for most infectious pathologies (S. pneumoniae, N. meningitidis, H. influenzae and S. aureus).
Type and period of the study
This Meta-analysis on antibiotic resistance in Morocco, national data on studies conducted in the last nine years (2011-2020).
Search strategy and criteria for choosing studies
Exploitation standards
Collecting articles for our meta-analysis using keywords: bacteria, antibiotic, resistance, S. pneumoniae, N. meningitidis, H. influenzae, and S. aureus. Searched for articles and studies in the literature regarding bacterial resistance to antibiotics carried out in Morocco between 2010 and 2020, and we made no distinction regarding the publication’s language.
Elimination standards
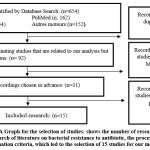
Published data on bacterial resistance to antibiotics, articles or studies that are on animals, outside the selected period, connected to our study but in different directions, and that are not on national data, were excluded. A meta-analysis was performed A meta-analysis was performed for 15 articles published using GraphPad Prisma 19.
Statistical analysis
For the statistical analysis, we used three different programs including: GraphPad Prisma 9 Review Manager 5.4 and Microsoft Excel, each of which has its own benefits and traits.
-GraphPad Prisma 9: for repeat and elimination criteria, and to graph years and regions of publication. A p value <0.05 was regarded as significant for statistical analyzes with a 95% confidence interval (95% CI)
-Review Manager 5.4: to compare similar studies.
-Microsoft Excel to create tables, collect and compare data.
Results and Discussions
Results
Studies published in the period of (2011-2020) in Moroccan regions on antibiotic resistance
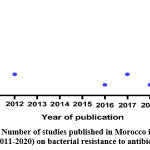 |
Figure 1: Number of studies published in Morocco in period of (2011-2020) on bacterial resistance to antibiotic. |
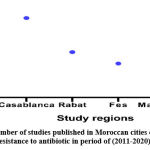 |
Figure 2: Number of studies published in Moroccan cities on bacterial resistance to antibiotic in period of (2011-2020). |
Studies characteristics that were considered in our meta-analysis
The 15 studies’ primary characteristics (in four Moroccan regions) within scope of this systematic review. Essentially all of these were based on laboratory controls, analytical research and diagnostic surveys as illustrated in (Figure 3).
Characteristics of the studies included in our analysis on antibiotic resistance
In each article, indicated the region and year of the study, the germs studied, the antibiotics, the most resistant bacteria, the number of references on which each research was based and the number of samples in each study. And we added comments for each reference.
Table 1: Summary of studies that were listed in our meta-analysis
| References | Year of publication | Region | N. of Samples | Microbe | Types of Antibiotics | Resistance | Comments |
| Bouskraoui et al 20 | 2011 | Marrakech | 302 | Streptococcus pneumoniae | PG | PG | penicillin resistance of carrier strains in children under 2 years old |
| N. Mdaghri et al.21 | 2012 | Casablanca | 185 | Neisseria meningitidis | PG | PG | In this study, the only antibiotic, penicillin G, gave significant resistance |
| Benbachir et al.22 | 2012 | Casablanca | 955 | Streptococcus pneumoniae | PNS, AMC, CRO, CHL, ERY, TET and TSU | PNS, AMC, CRO, ERY | Antibiotic resistance rates vary considerably by geographic location at MIC>2μg/ml |
| Diawara et al. 23 | 2016 | Casablanca | 655 | Streptococcus pneumoniae | MLSB | MLSB | Multi-resistant S.pneumoniae. Strains include both (vaccine and non-vaccine) serotypes. More observationnel studies are needed |
| Diawara et al.24 | 2017 | Casablanca | 361 | Streptococcus pneumoniae | PGand AMP | PG | The ratio was 22.2% overall for PG resistance in the research |
| Ghita Y et al. 25 | 2017 | Fes | 123 | Haemophilus influenzae | No resistance
sensitive |
sensible | H.influenzae, plays a major role in lower respiratory infections, these infections are a real public health problem. |
| Moumni M B et al.26 | 2018 | Fes | 277 | Neisseria meningitidis | CET | sensible | Following this study, we can conclude that the therapeutic regimens using 3rd generation cephalosporins in the treatment of community meningitis are effective. We also suggest the reinforcement of the vaccination program against H. influenzae b. |
| EL Amin G et al.,27 | 2019 | Rabat | 2436 | Streptococcus pneumoniae and Neisseria meningitidis | PG | PG | Nosocomial meningitis represents 73.8% of documented meningitis in this series. Many of them are related to neurosurgery or consecutive to the placement of a CSF shunt. |
Continue table (1): Summary of studies that were listed in our meta-analysis:
| References | Year of publication | Regions | N. of Samples | Microbe | Types of Antibiotics | Resistance | Comments |
| Rhars A et al. 28 | 2019 | Rabat | 100 | Staphylococcus aureus | PEN, CIP et ERY | PEN, CIP | The need to adapt probabilistic treatment regimens to the local epidemiology. |
| Kouara S et al. 29 | 2019 | Fes | 46 | Staphylococcus aureus | PEN, CIP et ERY | PEN, CIP, ERY | This class of antibiotics still retains its place in the treatment of staphylococci |
| Nzoyikorera N et al 30 | 2019 | Casablanca | 74 | Streptococcus pneumoniae | PG | PG | The data from this study on adult MS show variability in serotypes and provide information on the antibiotic susceptibility status of pneumococcus in adults |
|
Raghani A et al. 31 |
2019 | Rabat | 4232 | Streptococcus pneumoniae | PG | PG | The importance of the frequency of pneumopathies as well as the isolation of resistant germs incites us to improve the hygiene conditions, the means of prevention and the reasoned prescription of antibiotics |
|
Saoud M Z et al. 32 |
2019 | Rabat | 119 | Staphylococcus aureus | PEN, CIP et ERY | PEN, CIP et ERY |
All prescribed antibiotics were resisted by S. aureus |
| Ait Mouss et al. 33 | 2020 | Casablanca | 245 | Neisseria meningitidis | PG et 3GCs | PG |
All isolated strains are sensitive to third generation cephalosporin. |
| Ousaid et al. 3 | 2020 | Casablanca | 115 | Staphylococcus aureus | OXA, AMP, AMX,
CTX, SEF et ERY |
OXA, AMP, AMX, CTX et SEF | In Morocco and neighboring countries, the problem of antibiotic resistance is recognized and well documented. |
Abbreviations: PEN : penicillin, PG : Penicillin G, SEF : Cefpodoxime, CHL : Chloramphenicol, ERY : erythromycin, TET : Tetracyclin, OXA : Oxacillin, AMP : ampicillin, CIP : Ciprofloxacin, CTX : Cefotaxime, AMX : amoxicillin, CET : Ceftriaxon, MLSB : macrolide, lincosamides , streptogramin B et C3G : cephalosporins 3rd generation.
Epidemiological profile of invasive germs in the pediatric
Bacterial meningitis in the pediatric population remains a worrying pathology due to its frequency and severity, studies made on the antimicrobial resistance of the most frequent bacteria in hospitals in Morocco, and these studies have concluded that the most frequent bacteria: S. pneumoniae; N. meningitidis and H. influenzae.
According to these studies, which are presented in the table below, the prevalence of S. pneumoniae was 13%, N. meningitidis was 9.7%, and H. influenzae was 5.4% in Casablanca in 2012 (based on 185 samples).
In 2018, in Morocco, (out of 277 samples), S. pneumoniae infection was confirmed 29%, N. meningitidis infection was 33% and H. influenzae infection was 8%.
In 2016, a study was conducted in Rabat on 25 samples, half of the samples were infected with S. pneumoniae at 48%, N. meningitidis at 24% and H. influenzae at 8%.
According which studies (2012, 2018), ceftriaxone was used in the treatment in both studies and the outcome was favorable in all patients. Both studies concluded that C3Gs are the most effective treatment for bacterial meningitis and suggested that the vaccination program against H. Influenzae b should be strengthened.
Table 2: Epidemiological profile of invasive germs in the pediatric.
| References | Years | Regions | N. of samples | S. pneumoniae | N. meningitidis | H. influenzae |
| Mdaghri N, et al 2012 | 2012 | Casablanca | 185 | 13% | 9.7% | 5.4% |
| EL Amin G et al 2019 | 2016 | Rabat | 25 | 48% | 24% | 8% |
| Moumni M B,
et al 2018 |
2018 | Fes | 277 | 29% | 33% | 8% |
Evolution of S. pneumoniae resistance to antibiotics
The period of realization of the two studies is about seven years, where the resistance of bacteria was studied on both penicillin and erythromycin, at the beginning of the two studies the rate of bacterial resistance to penicillin was 25% and after about seven years was 31%.The rate of resistance to erythromycin was 15% and after seven years was 21%, therefore, there is a remarkable increase in bacterial resistance to the aforementioned antibiotics, which imposes attention to the use antibiotic in treatment S. pneumoniae infection and evaluation effectiveness treatment and antibiotic resistance heterogeneity.
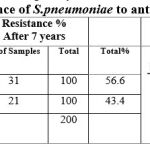 |
Table 3: Resistance of S.pneumoniae to antibiotics |
Resistance of S. pneumoniae to penicillin G
Through previous studies carried out from 2011 until 2020 on 302 samples, S. pneumoniae was the most resistant to Penicillin G, in 2011 the percentage of resistance to Penicillin G was at 2.7%, and in the second study carried out on 101 samples this resistance evolved to 100% in 2020.
Table 4: Resistance of S. pneumoniae to penicillin G: (SPRPG).
| References | Years | Regions | N. of samples | S. pneumoniae | RPG |
| Bouskraoui Met al 2011 | 2011 | Marrakech | 302 | 45.8% | 2.7% |
| Raghani A et al.2019 | 2012-2018 | Rabat | 4232 | 1% | 10.6% |
| Diawara et al, 2017 | 2014 | Casablanca | 361 | 100% | 22.2% |
| Nzoyikorera N et al.2019 | 2016-2018 | Casablanca | 74 | 32% | 18.75 |
| Ousaid et al, 2020 | 2020 | Casablanca | 101 | 86% | 100% |
Evolution of N.meningitidis resistance to penicillin G
Three studies on non-tuberculous bacterial meningitis in pediatric in two Moroccan regions (Casablanca and Fes), found that resistance of N. meningitidis to penicillin G was 4.3 in 2000, 11.1% in 2012, 16.6% in 2016, and 24% in 2019.
Table 5: Resistance of N. meningitidis to penicillin G: (NMRPG).
| References | Years | N. of samples | Regions | RPG |
| N. Mdaghri, et al 2012 | 2012 | 180/18 | Casablanca | 11.1% |
| EL Amin G et al 2019 | 2016 | 600/6 | Fes | 16.6% |
| K. Ait Mouss et al 2020 | 2019 | 245 | Casablanca | 24% |
Evolution of H. Influenzae resistance to antibiotics
Epidemiological profile of lower respiratory infections with H. influenzae
A study carried out in the period 2011-2016 on 123 strains of H. influenzae responsible for lower respiratory infections, this study showed that the strains that are resistant to amoxicillin are sensitive to the combination amoxicillin-clavulanic acid 28% of cases, and the resistance to other antibiotics 16% to trimethoprim -sulfamethoxazole, 4.8% to fluoroquinolones 2.5% to tetracyclins, and no resistance to 3rd generation cephalosporins was observed.
Table 6: Epidemiological profile of lower respiratory infections with H. influenzae.
| References | Years | Region | N. of Samples | RAMX | RTRM &RSU | RFLQ | RTET | C 3 G |
| Ghita Y et al 2017 | 2011 -2016 | Fes | 123 | S:AMC28 % | 16% | 4,8% | 2,5% | S |
Abbreviations: R: Resistance, S: Sensibles, AMX: amoxicillin, SU: sulfamethoxazole, FLQ: fluoroquinolones, TET: Tetracyclin, AMC: amoxicillin-clavulanic and TRM: The resistance of trimethoprim
Epidemiological profile meningitis of H. influenzae bacterial
The data of the retrospective studies carried out in Rabat over a period of three years based on CSF analysis of 95 positive cases were documented (59 adults and 36 children) of which the percentage of H. Influenzae cases was 8% (2 cases) and the antibiotic resistance was not documented, and in the second study carried out in Fes, showed that CSF analysis of 24 positive cases, of which the percentage of H. Influenzae cases was 8%, and the antibiotic resistance was not documented. It can be concluded that there is a lack of study of the resistance of H. Influenzae to antibiotics in these two studies Table 7.
Table 7: Epidemiological profile meningitis of H. influenzae bacterial
| References | Years | Regions | N. of Samples | H.influenzae |
| El amin G et al 2019 | 2016 – 2018 | Rabat | 600 | 8% |
| Moumni M B, et al 2018 | 2018 | Fes | 277 | 8% |
Evolution of S. aureus resistance to antibiotics:
S. aureus is the main cause of nosocomial infections and can affect several parts of the body and the emergence of resistant strains is a major problem in the public health of all the world that continues to increase according to the data of previous studies.
A study conducted over a period of 2016-2018 in Rabat, on 117 samples taken in consulting rooms and emergency cases with an average age of 47 years, where S. aureus was 92%, and tests were performed to determine its resistance to antibiotics, the percentage of resistance to penicillin G was 92%, to ciprofloxacin at 16.5%, and erythromycin at 14.6%.
Another study in Rabat conducted from 2017-2019 (Table 8) and the mean age of patients 49 year, and the samples were on patients with abscesses, diabetic foot and cellulitis, 29% of these samples were to S. aureus, and tests were performed showing that resistance to penicillin G was 89.6%, and to ciprofloxacin 6.9%, and resistance to erythromycin was not tested.
In another study conducted from 2018-2019 in Fes, on 46 samples of urine and venous catheters in people with an average age of 46 years, all samples contained S. aureus and were resistant to erythromycin to 88%, and ciprofloxacin 4% and penicillin G 2%.
Table 8: Resistance of S. aureus to antibiotics
| References | Years | Regions | Infectious pathologies | N. of Samples | RPENG | RCIP | RERY |
| Saoud M Z et al.2019 | 2016-2018 | Rabat | Surgical operations and emergencies | 117 | 92% | 16.51% | 14.61% |
| Rhars A et al 2019 | 2017-2019 | Rabat | Diabetic foot abscess and cellulitis | 100 | 89.6 | 6.9% | – |
| Kouara S et al 2019 | 2018-2019 | Fes | Intravenous catheters and urine cultures | 46 | 2% | 4% | 88% |
Abbreviations: PEN G: penicillin G, ERY: erythromycin CIP: ciprofloxacin.
Discussion
In our analysis of national data on bacterial resistance to antibiotics, we chosen 15 articles conducted over the previous ten years in four Moroccan regions, analyzed and compared the data from these studies to learn about antibiotic-resistant germs and the kinetics of this resistance, rapid emergence of resistant bacteria is occurring global, including in Morocco and neighboring countries, jeopardizing the effectiveness of antibiotics which have revolutionized medicine and let millions of people live longer 34. Misuse of these medications has been linked to the challenge of antibiotic resistance. In addition, paucity of development of new drugs due to weaker economic incentives and onerous regulatory requirements by the pharmaceutical industry, is a serious problem 35. The usage of antibiotics has a massive effect on these parameters, which has been extensively proven in Morocco 36.
Based our meta-analysis on the evaluation of the evolution of resistance of S. pneumoniae, N. meningitidis, H. influenzae, and S. aureus.
According the results obtained from the studies carried out in Morocco, we can say for the different bacteria, the prevalence of resistance increases with the years, in fact, in 2012 it was (13%, 9.7%, 5.4%) respectively S. pneumoniae, N. meningitidis and H. influenzae, in 2016 (48%, 24%, 8%) and in 2018 (29%, 33%, 8%), and we did not find documents on the prevalence of S.aureus.
We noticed that the evolution of resistance regarding S. pneumoniae was impacted by the introduction of the vaccine, rate of resistance S. pneumoniae to antibiotic erythromycin before vaccination was 76% and after the introduction of the vaccine decreased to 61%, and the incidence of pneumonia was 17.7% and after vaccination decreased to 10.2%. The resistance of S. pneumoniae to penicillin G, increased from 2.7% in 2011 to 100% in 2020,in addition, the study documented high rates of resistant to penicillin of S. pneumoniae in Spain [37], in Algeria, Egypt, Morocco and Senegal [3]. Increase in antibiotic resistance for S. pneumoniae has been attributed to several factors, differences in regulatory practices, economic factors and including sociocultural factors in France and Germany 37.
For N. meningitidis, there is an increase in resistance to penicillin G in Morocco where in 2012 was 11.1% to 24% in 2019. In USA the rate of resistance was 10.3% 36. N. meningitidis resistant to Penicillin G have become frequent and total resistance is increasing in Belgium to 4.8% 38,39, in Canada, 21.7% [40], and over the past 2 decades, an increase in penicillin G resistance has been observed in many parts of the world, with a higher rate in Europe 41,42. Penicillin resistance in N. meningitidis due to beta-lactamase production remains relatively rare. Isolates with resistance and reduced susceptibility to penicillin G due to alterations in the PENA gene (encoding penicillin binding protein 2) are reported, in 2016, a penicillin resistant clade of isolates MENW: CC11 with altered PENA genes was identified in Australia. most recent recently, increase in penicillin resistant invasive isolates of MENW:CC11 has been noted in England 43. Currently in France, the progressive, of the number of strains of decreased sensitivity with penicillins, the appearance of resistant strains to the penicillin G, make reconsider the problem of the sensitivity and resistance N. meningitidis to penicillin G 44.
Studies conducted from 2011 to 2016 in Morocco on H. influenzae resistance to antibiotics, we found that resistance for trimethoprim -sulfamethoxazole, fluoroquinolones, and tetracycline antibiotics is (16%, 4.8%, 2.5%) respectively, and that amoxicillin-resistant H. influenzae strains are susceptible to the combination of amoxicillin and clavulanic acid in 28% of cases and no resistance to C3Gs. Comparing the percentages for Qatar, Saudi Arabia and Islamic Republic of Iran, we found a high prevalence of β-lactam resistant isolates, respectively17.4% 45 ,43,6 %46, 34 % 47 and 35,7 % 48 and two studies conducted in Rabat and Fes in (2016-2018), it was noted that no antibiotic resistance was documented. Indeed, resistance to ampicillin by production of ß-lactamases Type TEM-1 (Exceptional ROB-1) concerns nearly 35% of H. influenzae strains in France. Decreased susceptibility by modification of the ß-lactam target is less frequent, reaching 8 to 10% of strains, but it can become a concern, affecting aminpenicillins and C3Gs to varying degrees, oral and injectable cephalosporins in Morocco 49, in France 50,51. in the United Kingdom and Germany 52,53 the antibiotic resistance is often multiple 37,54.
H. influenzae can develop resistance to fluoroquinolones through a typical stepwise mutation process of the primary target, DNA gyrase and topoisomerase IV, using four mutations in the GYRA genes, by C and by E. Initial treatment can also lead to GYRA mutations, with additional mutations occurring in Spain and France 55.
For the resistance of S.aureus in Morocco, it was found that the resistance increased significantly in 2016-2018 (Penicillin G 92%, ciprofloxacin 16.5% and erythromycin 14.6%) and in 2017-2019 the resistance to penicillin G 89.6%, ciprofloxacin 6.9% and erythromycin was not tested. In 2018 – 2019, (Penicillin G 2%, ciprofloxacin 4% and erythromycin 88%). These studies showed the percentage of resistant S.aureus.
In Ireland a study showed that 10 strains of S. aureus, (71.42%) were resistant to penicillin G, no strain was resistant to meticillin, gentamycin, rifampicin, teicoplanin or vancomycin 56. In USA today, more than 95% S. aureus is resistant to penicillin. Additionally, ampicillin and anti-pseudomonal penicillins 57.
The recommended treatment is generally betalactam (aminpenicillin or penicillin G) or a macrolide in case of allergy to penicillin in France, and in Belgium 58, in Canada It is noted that the incidence rate of S. aureus strains has gradually increased from 4.0% prior to 2000 to 5.2% in 2010-2020. It appears that this increasing rate is directly related to the increase in S. aureus infections and a change in antibiotic.59. In China, very low rates of resistance to penicillin G have been noted (11%) 60.
The results of meta-analysis on the evolution of the resistance of S. pneumoniae, N. meningitidis, H. influenzae, and S. aureus on the latest Moroccan national studies have shown the need for the implementation of a strategy national report on antibiotic resistant bacteria and that an effective plan is needed to combat this resistance.
Conclusion
This meta-analysis on the studies and researches on bacterial resistance to antibiotics in the last ten years in Morocco, described the incidence and the evolution of bacterial resistance of very frequent germs such as S. pneumoniae, N. meningitidis, H. influenzae and S. aureus reported between 2011-2020. It can be concluded that for the bacteria that we were interested in, the percentage of resistance varies for each species and that the incidence of resistance increases with the years. This increase has been attributed to several factors, include economic factors and socio-cultural, without neglecting role of antibiotic consumption, which has been amply demonstrated to fight against this resistance it is necessary to respect systematic surveillance measures, a good management the use of antibiotic as well as to respect rules and health protocol.
Competing interests
Authors declare that they have no competing interests. The views expressed in this paper are those of the authors and do not represent the official views of their organizations.
Funding Sources
This study did not receive any funding.
References
- OMS, “Organisation Mondiale de la Santé, Premier rapport de l’OMS sur la résistance aux antibiotiques: une menace grave d’ampleur mondiale 2014. Disponible sur: (http://www.who.int/mediacentre/news/releases/2014/amr-report/fr/,” OMS 2014.
- Abduladeem G. M. Al-Selwi and Amina. Barkat, “Antibiotic prescription in Morocco, national data: Meta-analysis,” Pharm. Negat. Results, vol. 13, no. 4, pp. 1074–1082, Nov. 2022, doi: 10.47750/pnr.2022.13.04.148.
- Ousaid, J. Akrim, and Y. Khayati, “Overuse of antibiotics as a key driver to antibiotic resistance in Morocco: A short review with potential solutions,” Int. Arab. J. Antimicrob. Agents, vol. 10, no. 1, Art. no. 1, May 2020, doi: 10.3823/843.
- Haskouri S, “résistance aux antibiotiques : mécanismes et évolution.,” université mohammed V faculté de médecine et de pharmacie de rabat, 2002.
- Weiss K, “la résistance bactérienne la nouvelle guerre froide,” Médecin Qué., vol. 37, n° 3, pp. 41–49, 2002.
- A. Saïd, “Evolution de la résistance bactérienne aux antibiotiques et conseils en antibiothérapie,” université mohammed V faculté de médecine et de pharmacie de rabat, 2016.
- Sekhsokh Yassine, “Résistance des bactéries aux antibiotiques dans le milieu extrahospitalier dans la ville de Kenitra.Theses:142. université Mohammed V faculté de médecine et de pharmacie de rabat,” 2017.
- M. . Morens, J. K. Taubenberger, and A. S. Fauci, “Predominant Role of Bacterial Pneumonia as a Cause of Death in Pandemic Influenza: Implications for Pandemic Influenza Preparedness,” J. Infect. Dis., vol. 198, no. 7, pp. 962–970, Oct. 2008, doi: 10.1086/591708.
- Kayemba‐Kay’s, A. M. Badran, C. Lagneaux, T. Kovacs, and A. Heron, “Streptococcus pneumoniae serotype 19A meningitis in well‐vaccinated immunocompetent 13‐month‐old child: a case of PCV13 failure,” Clin. Case Rep., vol. 4, no. 11, pp. 1023–1025, Sep. 2016, doi: 10.1002/ccr3.660.
- Lapeyssonnie and W. H. Organization, “La méningite cérébrospinale en Afrique,” Bull. World Health Organ. V 28 Suppl., 1963, Accessed: Nov. 06, 2021. [Online]. Available: https://apps.who.int/iris/handle/10665/72037
- Erickson and P. De Wals, “Complications and sequelae of meningococcal disease in Quebec, Canada, 1990-1994,” Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am., vol. 26, no. 5, pp. 1159–1164, May 1998, doi: 10.1086/520303.
- P. Rubach et al., “Increasing Incidence of Invasive Haemophilus influenzae Disease in Adults, Utah, USA – Volume 17, Number 9—September 2011 – Emerging Infectious Diseases journal – CDC,” 2011, doi: 10.3201/eid1709.101991.
- E. Shope, “SWINE INFLUENZA : III. FILTRATION EXPERIMENTS AND ETIOLOGY,” J. Exp. Med., vol. 54, no. 3, pp. 373–385, Jul. 1931, doi: 10.1084/jem.54.3.373.
- Dabernat H, “Données épidémiologiques de la résistance aux antibiotiques des Haemophilus, méningocoques, Listeria.,” Mdd Mal Infect, no. 32, pp. 299–306, 2002.
- Y. Liu et al., “Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity,” J. Exp. Med., vol. 202, no. 2, pp. 209–215, Jul. 2005, doi: 10.1084/jem.20050846.
- Goldstein FW, Pan Y, and Gertner J, “Resistance to ceftriaxone and other beta-lactams in bacteria isolated in the community. The Vigil’Roc Study Group – PubMed,” 1995. https://pubmed.ncbi.nlm.nih.gov/8585736/ (accessed May 22, 2021).
- Aboya Moroh J-L, “Résistance bactérienne et phytomolécules antimicrobiennes issues de Morinda morindoides. Agricultural sciences. Université de Bretagne occidentale – Brest,” Université Félix Houphouët- Boigny, French, 2013.
- Henriet and D. Guillemot, “Pharmaco-épidémiologie des résistances, consommation des antibiotiques,” Médecine Mal. Infect., vol. 30, pp. s160–s163, May 2000, doi: 10.1016/S0399-077X(00)89084-8.
- Moher, A. Liberati, J. Tetzlaff, D. G. Altman, and PRISMA Group, “Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement,” Int. J. Surg. Lond. Engl., vol. 8, no. 5, pp. 336–341, 2010, doi: 10.1016/j.ijsu.2010.02.007.
- Bouskraoui et al., “Étude du portage rhinopharyngé de Streptococcus pneumoniae et de sa sensibilité aux antibiotiques chez les enfants en bonne santé âgés de moins de 2 ans dans la région de Marrakech (Maroc),” Arch. Pédiatrie, vol. 18, no. 12, pp. 1265–1270, 2011.
- Mdaghri, N. Jilali, H. Belabbes, Z. Jouhadi, M. Lahssoune, and S. Zaid, “Epidemiological profile of invasive bacterial diseases in children in Casablanca, Morocco: Antimicrobial susceptibilities and serotype distribution,” East. Mediterr. Health J. Rev. Santé Méditerranée Orient. Al-Majallah Al-Ṣiḥḥīyah Li-Sharq Al-Mutawassiṭ, vol. 18, pp. 1097–101, Nov. 2012, doi: 10.26719/2012.18.11.1097.
- Benbachir, N. Elmdaghri, H. Belabbes, G. Haddioui, H. Benzaid, and B. Zaki, “Eleven-year surveillance of antibiotic resistance in Streptococcus pneumoniae in Casablanca (Morocco),” Microb. Drug Resist., vol. 18, no. 2, pp. 157–160, 2012.
- Diawara et al., “Phenotypic and genotypic characterization of Streptococcus pneumoniae resistant to macrolide in Casablanca, Morocco,” Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis., vol. 40, pp. 200–204, Jun. 2016, doi: 10.1016/j.meegid.2016.03.003.
- Diawara et al., “Molecular characterization of penicillin non-susceptible Streptococcus pneumoniae isolated before and after pneumococcal conjugate vaccine implementation in Casablanca, Morocco,” Ann. Clin. Microbiol. Antimicrob., vol. 16, no. 1, p. 23, Dec. 2017, doi: 10.1186/s12941-017-0200-6.
- Ghita Y, Rajae, and Mustapha M, “Épidémiologie et évaluation de la sensibilité des souches d’Haemophilus influenzae isolées d’infections des voies respiratoires basses,” J. Innov. Appl. Stud., vol. 20, no. 2028–9324, pp. 787–791, 2017.
- MOUMNI M B, YAHYAOUI G, and MAHMOUD M, “MENINGITES PURULENTES EN MILIEU PEDIATRIQUE : EXPERIENCE DU LABORATOIRE CENTRAL DU CHU HASSAN II DE FES,” Société Marocaine Microbiol. Médicale, 2016.
- L Amin G et al., “Profil Epidemiologique des meningites bacteriennes,” Société Marocaine Microbiol. Médicale, p. P045, 2019.
- Rhars A et al., “Epidemiologie bacterienne des infections des parties molles,” Société Marocaine Microbiol Médicale, p. 11, 2019.
- Kouara S, Filali M, Samouche M, Yahyaoui G, and Mahmoud M, “Profil Bactériologique des infections a Staphylocuccus aureus en milieu pédiatrique au chu de fè,” Société Marocaine Microbiol. Médicale, p. P067, 2019.
- Nzoyikorera N et al., “Epidemiologie des infections invasives a streptococcus pneumoniae chez l’adulte a casablanca entre 2016-2018,” Société Marocaine Microbiol. Médicale, p. P076, 2019.
- Raghani A et al., “Epidemiologie bacterienne des pneumopathies : etude retrospective sur 7 ans,” Société Marocaine Microbiol. Médicale, p. P079Bis, 2019.
- Saoud M Z et al., “Infections profondes a staphylococcus aureus : à propos de 117 cas,” Société Marocaine Microbiol. Médicale, p. P086, 2019.
- Ait Mouss et al., “Epidemiological profile of Neisseria meningitidis in Casablanca, Morocco: 2010-2019,” Access Microbiol., vol. 2, no. 9, p. acmi000157, 2020, doi: 10.1099/acmi.0.000157.
- A. Moghnieh, Z. A. Kanafani, H. Z. Tabaja, S. L. Sharara, L. S. Awad, and S. S. Kanj, “Epidemiology of common resistant bacterial pathogens in the countries of the Arab League,” Lancet Infect. Dis., vol. 18, no. 12, pp. e379–e394, Dec. 2018, doi: 10.1016/S1473-3099(18)30414-6.
- A. Michael, D. Dominey-Howes, and M. Labbate, “The Antimicrobial Resistance Crisis: Causes, Consequences, and Management,” Front. Public Health, vol. 2, Sep. 2014, doi: 10.3389/fpubh.2014.00145.
- Brian H. Harcourt et al., “Population-Based Surveillance of Neisseria meningitidis Antimicrobial Resistance in the United States,” Open Forum Infect. Dis., vol. 2, no. 3, 2015, doi: https://doi.org/10.1093/ofid/ofv117.
- Fenoll, I. Jado, D. Vicioso, A. Pérez, and J. Casal, “Evolution of Streptococcus pneumoniaeSerotypes and Antibiotic Resistance in Spain: Update (1990 to 1996),” J. Clin. Microbiol., vol. 36, no. 12, pp. 3447–3454, Dec. 1998, doi: 10.1128/JCM.36.12.3447-3454.1998.
- SBIMC, “Recommandations de traitements anti-infectieux en milieu hospitalier.”
- Bertrand, F. Carion, R. Wintjens, V. Mathys, and R. Vanhoof, “Evolutionary changes in antimicrobial resistance of invasive Neisseria meningitidis isolates in Belgium from 2000 to 2010: increasing prevalence of penicillin nonsusceptibility,” Antimicrob. Agents Chemother., vol. 56, no. 5, pp. 2268–2272, May 2012, doi: 10.1128/AAC.06310-11.
- E. Rosenstein, S. A. Stocker, T. Popovic, F. C. Tenover, and B. A. Perkins, “Antimicrobial resistance of Neisseria meningitidis in the United States, 1997. The Active Bacterial Core Surveillance (ABCs) Team,” Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am., vol. 30, no. 1, pp. 212–213, Jan. 2000, doi: 10.1086/313599.
- Muhamed-Kheir Taha, Julio A Vázquez, Eva Hong, Desiree E Bennett, and Sophie Bertrand, “Target gene sequencing to characterize the penicillin G susceptibility of Neisseria meningitidis,” Antimicrob Agents Chemother, vol. 51, no. 8, pp. 2784–92, 2007, doi: doi: 10.1128/AAC.00412-07.
- Merijn W Bijlsma, Vincent Bekker, Matthijs C Brouwer, Lodewijk Spanjaard, Diederik van de Beek, and Arie van der Ende, “Epidemiology of invasive meningococcal disease in the Netherlands, 1960-2012: an analysis of national surveillance data,” Lancet Infect Dis, vol. 14, no. 9, pp. 805–12, 2014, doi: doi: 10.1016/S1473-3099(14)70806-0.
- Willerton et al., “Increase in penicillin-resistant invasive meningococcal serogroup W ST-11 complex isolates in England,” Vaccine, vol. 39, no. 19, pp. 2719–2729, May 2021, doi: 10.1016/j.vaccine.2021.03.002.
- Cavallo JD, Nicolas P, and Martet G, “Actualités sur la sensibilité de Neisseria meningitidis aux antibiotiques et en particulier aux bêtalactamines. La Lettre de l’Infectiologue.,” Tome XIII, vol. 9, pp. 429–433, 1998.
- Kassaa, M. Hamze, F. Dabboussi, H. Mallat, M. Achkar, and S. Hlais, “Prevalence of type b Haemophilus influenzae and antibiotic resistance in 52 clinical isolates in north Lebanon,” East. Mediterr. Health J. Rev. Santé Méditerranée Orient., vol. 19, p. 108, 2013.
- Mojgani, M. Rahbar, M. Taqizadeh, M. P. Ashtiani, and M. Mohammadzadeh, “Biotyping, capsular typing, and antibiotic resistance pattern of Haemophilus influenzae strains in Iran,” Jpn. J. Infect. Dis., vol. 64, no. 1, pp. 66–68, 2011.
- Al-Binali and S. Al-Fifi, “Profile of childhood meningitis in a hospital in South West Saudi Arabia.,” Saudi Med. J., 2002.
- F. Elsaid et al., “Clinical presentation of acute bacterial meningitis in Qatar,” Neurosci. Riyadh Saudi Arab., vol. 7, no. 4, pp. 266–271, Oct. 2002.
- Dabernat H., “Haemophilus influenzae. (Centre national de référence des Haemophilus influenzae). Surveillance nationale des maladies infectieuses 1998–2000. Saint-Maurice,” Veille- Sanit., pp. 91–5, 2000.
- Dabernat, M. Seguy, G. Faucon, and C. Delmas, “Épidémiologie et évaluation de la sensibilité aux antibiotiques de souches d’Haemophilus influenzae isolées en 2004 en France,” 2007, doi: 10.1016/J.MEDMAL.2006.10.014.
- Masson, “Activité de 9 -lactamines sur 280 souches d’Haemophilus influenzae résistantes à l’ampicilline par bêtalactamase et mécanisme non enzymatique,” EM-Consulte, 2022. https://www.em-consulte.com/article/164351/activite-de-9-lactamines-sur-280-souches-d-haemoph (accessed Apr. 26, 2022).
- Ball et al., “Antibiotic therapy of community respiratory tract infections: strategies for optimal outcomes and minimized resistance emergence,” J. Antimicrob. Chemother., vol. 49, no. 1, pp. 31–40, Jan. 2002, doi: 10.1093/jac/49.1.31.
- Lode and M. Allewelt, “Role of newer fluoroquinolones in lower respiratory tract infections,” J. Antimicrob. Chemother., vol. 49, no. 5, pp. 709–712, May 2002, doi: 10.1093/jac/dkf024.
- A. Needham, “Haemophilus influenzae: antibiotic susceptibility.,” Clin. Microbiol. Rev., vol. 1, no. 2, pp. 218–227, Apr. 1988.
- Teresa Bastida et al., “Levofloxacin treatment failure in Haemophilus influenzae pneumonia – PubMed,” 2003. https://pubmed.ncbi.nlm.nih.gov/14718097/ (accessed Apr. 07, 2022).
- Maleb, M. Frikh, Y. B. Lahlou, B. Belefquih, A. Lemnouer, and M. Elouennass, “Écoulements vaginaux d’origine infectieuse chez la femme adulte à l’hôpital militaire d’instruction Mohammed V de Rabat (Maroc) : étude de 412 cas,” Rev. Sage-Femme, vol. 17, no. 3, pp. 122–126, Jun. 2018, doi: 10.1016/j.sagf.2018.03.004.
- J. Foster, “Antibiotic resistance in Staphylococcus aureus. Current status and future prospects,” FEMS Microbiol. Rev., vol. 41, no. 3, pp. 430–449, May 2017, doi: 10.1093/femsre/fux007.
- Bergogne-B, “Flores vaginales normales, vaginites et vaginoses bactériennes : diagnostic et thérapeutique,” Flores Vaginales Norm. Vaginites Vaginoses Bactériennes Diagn. Thérapeutique, vol. 9, no. 2, pp. 139–144, 2007.
- Castanheira, A. A. Watters, R. E. Mendes, D. J. Farrell, and R. N. Jones, “Occurrence and molecular characterization of fusidic acid resistance mechanisms among Staphylococcus spp. from European countries (2008),” J. Antimicrob. Chemother., vol. 65, no. 7, pp. 1353–1358, Jul. 2010, doi: 10.1093/jac/dkq094.
- Jeffery Ho, Maureen V Boost, and Margaret M O’Donoghue, “Tracking sources of Staphylococcus aureus hand contamination in food handlers by spa typing – PubMed,” 2015. https://pubmed.ncbi.nlm.nih.gov/25997877/ (accessed Apr. 09, 2022).