Article contents
Effect of Exchangeable Cation on X-Ray Diffraction Patterns and Thermal Behavior of a Montmorillonite Clay
Published online by Cambridge University Press: 01 January 2024
Abstract
A stratum of bentonite in the North Park (?) formation near Granby, Colorado, is composed largely of a dioctahedral Ca-montmorillonite whose formula is calculated to be $\left( {A{l_{2.84}}F{e_{0.50}}M{g_{0.72}}M{n_{0.04}}} \right)\left( {\mathop {A{l_{0.40}}S{i_{7.60}}}\limits^{\mathop \uparrow \limits^{{X_{0.86}}} } } \right){O_{20}}{\left( {OH} \right)_4}.$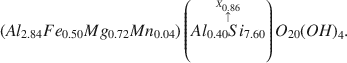
Na+, K+, Li+, H+, NH4+, Ca++, and Mg++ modifications were stored at 52 percent relative humidity and at 105° C-110° C. Results of X-ray diffraction, differential thermal, and thermal balance analysis depend upon the exchangeable cation and prior treatment. As with many montmorillonoids, d(001) = 22.7−30.1 Å under room conditions; ao = 5.20 Å and b0= 9.00 Å. The (001 ) interference indicates that the unit cell typically includes two packets, or possibly more, which may be derived geometrically from each other by a glide of 1.73 Å along (110) and 180° rotation. Weight loss above 190° C-3670 C exceeds that indicated by the Hofmann structure but conforms reasonably with loss indicated by a structure after that proposed by Edelman. Inverted Si-O tetrahedra are presumed to equal the number of univalent cations
It is suggested that the exchangeable cations form hydroxides during thermal analysis by reaction with (OH)− at the apex of inverted Si-0 tetrahedra. The resulting H2O and NH4OH are lost during thermal analysis, thus explaining excessive weight loss. Ca (OH)2 and Mg(OH)2 so produced release one mole of H2O during thermal analysis. KOH, NaOH, and LiOH are not decomposed below 1,000° C.
Thermal products vary with exchangeable cation and crystallinity increases with prior drying. The Li+ and Ca++ modifications produce beta-quartz and alpha-cristobalite with spinel and glass, whereas the other modifications produce only spinel and glass.
- Type
- Article
- Information
- Clays and Clay Minerals (National Conference on Clays and Clay Minerals) , Volume 3 , February 1954 , pp. 146 - 173
- Copyright
- Copyright © The Clay Minerals Society 1954
References
- 11
- Cited by




