Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Turner, David R.
Pabalan, Roberto T.
Prikryl, James D.
and
Paul Bertetti, F.
1999.
Radionuclide Sorption at Yucca Mountain, Nevada - A Demonstration of an Alternative Approach for Performance Assessment.
MRS Proceedings,
Vol. 556,
Issue. ,
Turner, David R.
and
Pabalan, Roberto T.
1999.
Abstraction of mechanistic sorption model results for performance assessment calculations at Yucca Mountain, Nevada.
Waste Management,
Vol. 19,
Issue. 6,
p.
375.
Misaelides, P.
and
Godelitsas, A.
1999.
Natural Microporous Materials in Environmental Technology.
p.
193.
Contardi, J.S.
Turner, D.R.
and
Ahn, T.M.
2001.
Modeling colloid transport for performance assessment.
Journal of Contaminant Hydrology,
Vol. 47,
Issue. 2-4,
p.
323.
Silva, Robert J.
and
Nitsche, Heino
2001.
Environmental Actinide Science.
MRS Bulletin,
Vol. 26,
Issue. 9,
p.
707.
Wang, Peiming
Anderko, Andrzej
and
Turner, David R.
2001.
Thermodynamic Modeling of the Adsorption of Radionuclides on Selected Minerals. I: Cations.
Industrial & Engineering Chemistry Research,
Vol. 40,
Issue. 20,
p.
4428.
Wang, Peiming
Anderko, Andrzej
and
Turner, David R.
2001.
Thermodynamic Modeling of the Adsorption of Radionuclides on Selected Minerals. II: Anions.
Industrial & Engineering Chemistry Research,
Vol. 40,
Issue. 20,
p.
4444.
Painter, S.
Cvetkovic, V.
and
Turner, D.R.
2001.
Effect of Heterogeneity on Radionuclide Retardation in the Alluvial Aquifer Near Yucca Mountain, Nevada.
Groundwater,
Vol. 39,
Issue. 3,
p.
326.
Kraemer, Stephan M.
Xu, Jide
Raymond, Kenneth N.
and
Sposito, Garrison
2002.
Adsorption of Pb(II) and Eu(III) by Oxide Minerals in the Presence of Natural and Synthetic Hydroxamate Siderophores.
Environmental Science & Technology,
Vol. 36,
Issue. 6,
p.
1287.
Runde, Wolfgang
Conradson, Steve D
Wes Efurd, D
Lu, NingPing
VanPelt, Craig E
and
Tait, C.Drew
2002.
Solubility and sorption of redox-sensitive radionuclides (Np, Pu) in J-13 water from the Yucca Mountain site: comparison between experiment and theory.
Applied Geochemistry,
Vol. 17,
Issue. 6,
p.
837.
Coppin, Frédéric
Berger, Gilles
Bauer, Andreas
Castet, Sylvie
and
Loubet, Michel
2002.
Sorption of lanthanides on smectite and kaolinite.
Chemical Geology,
Vol. 182,
Issue. 1,
p.
57.
Siegel, M.D.
and
Bryan, C.R.
2003.
Treatise on Geochemistry.
p.
205.
Bourg, Ian C.
Bourg, Alain C.M.
and
Sposito, Garrison
2003.
Modeling diffusion and adsorption in compacted bentonite: a critical review.
Journal of Contaminant Hydrology,
Vol. 61,
Issue. 1-4,
p.
293.
Moore, Robert C.
Holt, Kathleen
Zhao, Hongting
Hasan, Ahmed
Awwad, Nasser
Gasser, Mona
and
Sanchez, Charles
2003.
Sorption of Np(V) by synthetic hydroxyapatite.
Radiochimica Acta,
Vol. 91,
Issue. 12,
p.
721.
Coppin, Frédéric
Castet, Sylvie
Berger, Gilles
and
Loubet, Michel
2003.
Microscopic reversibility of Sm and Yb sorption onto smectite and kaolinite.
Geochimica et Cosmochimica Acta,
Vol. 67,
Issue. 14,
p.
2515.
Rodrigues, M. G. F.
Silva, M. L. P.
and
Silva, M. G. C. da
2004.
Caracterização da argila bentonítica para utilização na remoção de chumbo de efluentes sintéticos.
Cerâmica,
Vol. 50,
Issue. 315,
p.
190.
Yamaguchi, Tetsuji
Nakayama, S.
and
Yoshida, T.
2004.
Interactions between anionic complex species of actinides and negatively charged mineral surfaces.
Radiochimica Acta,
Vol. 92,
Issue. 9-11,
p.
677.
Duff, M. C.
Hunter, D. B.
Hobbs, D. T.
Fink, S. D.
Dai, Z.
and
Bradley, J. P.
2004.
Mechanisms of Strontium and Uranium Removal from High-Level Radioactive Waste Simulant Solutions by the Sorbent Monosodium Titanate.
Environmental Science & Technology,
Vol. 38,
Issue. 19,
p.
5201.
Del Nero, Mirella
Assada, Aminou
Madé, Benoit
Barillon, Rémi
and
Duplâtre, Gilles
2004.
Surface charges and Np(V) sorption on amorphous Al and Fe silicates.
Chemical Geology,
Vol. 211,
Issue. 1-2,
p.
15.
Cvetkovic, V.
Painter, S.
Turner, D.
Pickett, D.
and
Bertetti, P.
2004.
Parameter and model sensitivities for colloid‐facilitated radionuclide transport on the field scale.
Water Resources Research,
Vol. 40,
Issue. 6,
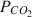 on Np(V) sorption on SAz-1 montmorillonite. The results show that Np(V) sorption on montmorillonite is strongly influenced by pH and PCO2
on Np(V) sorption on SAz-1 montmorillonite. The results show that Np(V) sorption on montmorillonite is strongly influenced by pH and PCO2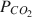 . In the absence of CO2, Np(V) sorption increases over the entire pH range examined (∼3 to ∼10), with measured sorption coefficients (KD) of about 10 mL g−1 at pH < 6 to KD ∼ 1000 mL g−1 at a pH of 10.5. However, for experiments open to atmospheric CO2 (PCO2=10−3.5atm
. In the absence of CO2, Np(V) sorption increases over the entire pH range examined (∼3 to ∼10), with measured sorption coefficients (KD) of about 10 mL g−1 at pH < 6 to KD ∼ 1000 mL g−1 at a pH of 10.5. However, for experiments open to atmospheric CO2 (PCO2=10−3.5atm ), Np(V) sorption peaks at KD ∼ 100 mL g−1 at pH of 8 to 8.5 and decreases at higher or lower pH. A comparison of the pH-dependence of Np(V) sorption with that of Np(V) aqueous speciation indicates a close correlation between Np(V) sorption and the stability field of the Np(V)-hydroxy complex NpO2OH0 (aq). In the presence of CO2 and aqueous carbonate, sorption is inhibited at pH > 8 due to formation of aqueous Np(V)-carbonate complexes. A relatively simple 2-site Diffuse-Layer Model (DLM) with a single Np(V) surface complexation reaction per site effectively simulates the complex sorption behavior observed in the Np(V)-H2O-CO2-montmorillonite system. The good agreement between measured and DLM-predicted sorption values suggests that surface complexation models based on parameters derived from a limited set of data could be useful in extrapolating radionuclide sorption over a range of geochemical conditions. Such an approach could be used to support transport modeling and could provide a better alternative to the current use of constant KD values in performance assessment transport calculations.
), Np(V) sorption peaks at KD ∼ 100 mL g−1 at pH of 8 to 8.5 and decreases at higher or lower pH. A comparison of the pH-dependence of Np(V) sorption with that of Np(V) aqueous speciation indicates a close correlation between Np(V) sorption and the stability field of the Np(V)-hydroxy complex NpO2OH0 (aq). In the presence of CO2 and aqueous carbonate, sorption is inhibited at pH > 8 due to formation of aqueous Np(V)-carbonate complexes. A relatively simple 2-site Diffuse-Layer Model (DLM) with a single Np(V) surface complexation reaction per site effectively simulates the complex sorption behavior observed in the Np(V)-H2O-CO2-montmorillonite system. The good agreement between measured and DLM-predicted sorption values suggests that surface complexation models based on parameters derived from a limited set of data could be useful in extrapolating radionuclide sorption over a range of geochemical conditions. Such an approach could be used to support transport modeling and could provide a better alternative to the current use of constant KD values in performance assessment transport calculations.



