Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Petit, S.
Righi, D.
and
Decarreau, A.
2008.
Transformation of Synthetic Zn-Stevensite to Zn-Talc Induced by the Hofmann-Klemen Effect.
Clays and Clay Minerals,
Vol. 56,
Issue. 6,
p.
645.
Andrieux, P.
and
Petit, S.
2010.
Hydrothermal synthesis of dioctahedral smectites: The Al–Fe3+ chemical series.
Applied Clay Science,
Vol. 48,
Issue. 1-2,
p.
5.
Le Forestier, Lydie
Muller, Fabrice
Villieras, Frédéric
and
Pelletier, Manuel
2010.
Textural and hydration properties of a synthetic montmorillonite compared with a natural Na-exchanged clay analogue.
Applied Clay Science,
Vol. 48,
Issue. 1-2,
p.
18.
Madejová, Jana
Pálková, Helena
and
Komadel, Peter
2010.
Spectroscopic Properties of Inorganic and Organometallic Compounds.
p.
22.
Vingiani, S.
Terribile, F.
Meunier, A.
and
Petit, S.
2010.
Weathering of basaltic pebbles in a red soil from Sardinia: A microsite approach for the identification of secondary mineral phases.
CATENA,
Vol. 83,
Issue. 2-3,
p.
96.
Wiseman, Sandra M.
Arvidson, R. E.
Morris, R. V.
Poulet, F.
Andrews‐Hanna, J. C.
Bishop, J. L.
Murchie, S. L.
Seelos, F. P.
Des Marais, D.
and
Griffes, J. L.
2010.
Spectral and stratigraphic mapping of hydrated sulfate and phyllosilicate‐bearing deposits in northern Sinus Meridiani, Mars.
Journal of Geophysical Research: Planets,
Vol. 115,
Issue. E7,
Scott, Bradley
Crouse, Jeff
Correia, Maria
Sun, Laisheng
and
Villemure, Gilles
2010.
Preparation of well-ordered hexagonal particles of synthetic cobalt smectites containing electrochemically active cobalt(II) sites.
Applied Clay Science,
Vol. 48,
Issue. 1-2,
p.
46.
Neumann, Anke
Petit, Sabine
and
Hofstetter, Thomas B.
2011.
Evaluation of redox-active iron sites in smectites using middle and near infrared spectroscopy.
Geochimica et Cosmochimica Acta,
Vol. 75,
Issue. 9,
p.
2336.
Neumann, Anke
Sander, Michael
and
Hofstetter, Thomas B.
2011.
Aquatic Redox Chemistry.
Vol. 1071,
Issue. ,
p.
361.
Decarreau, Alain
Vigier, Nathalie
Pálková, Helena
Petit, Sabine
Vieillard, Philippe
and
Fontaine, Claude
2012.
Partitioning of lithium between smectite and solution: An experimental approach.
Geochimica et Cosmochimica Acta,
Vol. 85,
Issue. ,
p.
314.
Thollot, Patrick
Mangold, Nicolas
Ansan, Véronique
Le Mouélic, Stéphane
Milliken, Ralph E.
Bishop, Janice L.
Weitz, Catherine M.
Roach, Leah H.
Mustard, John F.
and
Murchie, Scott L.
2012.
Most Mars minerals in a nutshell: Various alteration phases formed in a single environment in Noctis Labyrinthus.
Journal of Geophysical Research: Planets,
Vol. 117,
Issue. E11,
Lascelles, D. F.
2013.
Plate tectonics caused the demise of banded iron formations.
Applied Earth Science,
Vol. 122,
Issue. 4,
p.
230.
Bishop, Janice L.
Loizeau, Damien
McKeown, Nancy K.
Saper, Lee
Dyar, M. Darby
Des Marais, David J.
Parente, Mario
and
Murchie, Scott L.
2013.
What the ancient phyllosilicates at Mawrth Vallis can tell us about possible habitability on early Mars.
Planetary and Space Science,
Vol. 86,
Issue. ,
p.
130.
Petit, S.
and
Madejova, J.
2013.
Handbook of Clay Science.
Vol. 5,
Issue. ,
p.
213.
Bost, Nicolas
Westall, Frances
Ramboz, Claire
Foucher, Frédéric
Pullan, Derek
Meunier, Alain
Petit, Sabine
Fleischer, Iris
Klingelhöfer, Göstar
and
Vago, Jorge L.
2013.
Missions to Mars: Characterisation of Mars analogue rocks for the International Space Analogue Rockstore (ISAR).
Planetary and Space Science,
Vol. 82-83,
Issue. ,
p.
113.
Baker, Leslie L.
and
Strawn, Daniel G.
2014.
Temperature Effects on the Crystallinity of Synthetic Nontronite and Implications for Nontronite Formation in Columbia River Basalts.
Clays and Clay Minerals,
Vol. 62,
Issue. 2,
p.
89.
Baldermann, A.
Dohrmann, R.
Kaufhold, S.
Nickel, C.
Letofsky-Papst, I.
and
Dietzel, M.
2014.
The Fe-Mg-saponite solid solution series – a hydrothermal synthesis study.
Clay Minerals,
Vol. 49,
Issue. 3,
p.
391.
Mano, Eliana Satiko
Caner, Laurent
Petit, Sabine
Chaves, Arthur Pinto
and
Mexias, André Sampaio
2014.
Mineralogical Characterization of Ni-Bearing Smectites from Niquelândia, Brazil.
Clays and Clay Minerals,
Vol. 62,
Issue. 4,
p.
324.
Liu, Renlan
Xiao, Dongxue
Guo, Yaoguang
Wang, Zhaohui
and
Liu, Jianshe
2014.
A novel photosensitized Fenton reaction catalyzed by sandwiched iron in synthetic nontronite.
RSC Advances,
Vol. 4,
Issue. 25,
p.
12958.
Decarreau, A.
and
Petit, S.
2014.
Fe3+/Al3+ partitioning between tetrahedral and octahedral sites in dioctahedral smectites.
Clay Minerals,
Vol. 49,
Issue. 5,
p.
657.
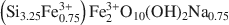 . A strictly ferric end-member of the nontronite series was therefore synthesized for the first time. The uncommon chemistry of the synthesized nontronites, notably the high level of Fe-for-Si substitution, induced particular XRD, FTIR, and differential thermal analysis-thermogravimetric analysis data. The ethylene glycol expandability of the synthetic nontronites was linked to their crystallinity and depended on the nature of the interlayer cation, moving from smectite to vermiculite-like behavior. As the synthesis temperature increased, the crystallinity of the synthesized clays increased. The nontronite obtained at 150°C had the ‘best crystallinity’, which cannot be improved by increasing synthesis time or temperature.
. A strictly ferric end-member of the nontronite series was therefore synthesized for the first time. The uncommon chemistry of the synthesized nontronites, notably the high level of Fe-for-Si substitution, induced particular XRD, FTIR, and differential thermal analysis-thermogravimetric analysis data. The ethylene glycol expandability of the synthetic nontronites was linked to their crystallinity and depended on the nature of the interlayer cation, moving from smectite to vermiculite-like behavior. As the synthesis temperature increased, the crystallinity of the synthesized clays increased. The nontronite obtained at 150°C had the ‘best crystallinity’, which cannot be improved by increasing synthesis time or temperature.



