Abstract
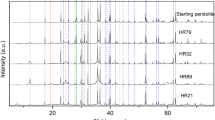
Todorokite commonly occurs in Earth surface environments. The factors governing formation of todorokite, such as reaction temperature, metal ions, dissolved O2 and pH, were investigated in this paper. Results showed that the forming rate of todorokite and its crystallinity decreased with falling reaction temperature, and the effect of temperature was more significant than that of other parameters. Nature of metal ions in the interlayer of buserite precursor and the structure of the buserite precursor obviously affected buserite transformation into todorokite. Weak bonding between the metal ions and MnO6 layer of buserite was favorable to todorokite formation. The rate of todorokite formation was promoted at a lower temperature with appropriate bubbling of O2. The pH in the system slightly influenced the todorokite formation, and todorokite could also be formed in a weak alkali medium or in a slightly acidic medium. Aged buserite precursor more easily form todorokite than the unaged one.
Similar content being viewed by others
References
Shen, Y. F., Zerger, R. P., Suib, S. L. et al., Manganese oxide octahedral molecular sieves: preparation, characterization and application, Science, 1993, 260: 511–515.
Post, J. E., Manganese oxide minerals: Crystal structures and economic and environmental significance, Proceedings National Academy of Science, 1999, 96: 3447–3454.[DOI]
Liu, F., Tan, W. F., Liu, G. Q. et al., Adsorption of heavy metal ions on Fe-Mn nodules in several soils and types of Mn oxide minerals, Acta Pedol Sinica (in Chinese with English abstracts), 2000, 39(5): 699–706.
Kumagai, N., Komaba, S., Sakai, H. et al., Preparation of to-dorokite-type manganese-based oxide and its application as lithium and magnesium rechargeable battery cathode, Journal of Power Sources, 2001, 97–98. 515–517.[DOI]
Al-Sagheer, F. A., Zaki, M. L., Synthesis and surface characterization of todorokite-type microporous manganese oxides: implications for shape-selective oxidation catalysts, Microporous and Mesoporous Materials, 2004, 67: 43–52.[DOI]
Golden, D. C., Chen, C. C., Dixon, J. B., Synthesis of todorokite, Science, 1986, 231: 717–719.
Dyer, A., Pillinger, M., Newton, J. et al., Sorption behavior of ra-dionuclides on crystalline synthetic tunnel manganese oxides, Chemistry of Materials, 2000, 12: 3798–3804.[DOI]
Feng, X. H., Liu, F., Tan, W. F. et al., Synthesis of todorokite by refluxing process and its primary characteristics, Science in China, Ser. D, 2004, 47(8): 760–768. [Abstract]
Feng, X. H., Tan, W. F., Liu, F. et al., Synthesis of todorokite at atmospheric pressure, Chemistry of Materials, 2004, 16: 4330–4336.[DOI]
Feng, X. H., Tan, W. F., Liu, F. et al., Hydrothermal synthesis of todorokite and its influencing factors, Earth Science-J. China University of Geosciences (in Chinese with English abstracts), 2005, 30(3): 347–352.
Turner, S., Siegel, M. D., Buseck, P. R., Structure features of to-dorokite intergrowths in manganese nodules, Nature, 1982, 296: 841–842.[DOI]
Lei, G., Crystal structures and metal uptake capacity of 10Å-manganates: an overview, Marine Geology, 1996, 133: 103–112.[DOI]
Tan, W. F., Liu, F., Li, Y. H. et al., Mineralogy of manganese in iron-manganese nodules of several soils in China, Acta Pedol. Sinica (in Chinese with English abstracts), 2000, 37(2): 192–201.
Giovanoli, R., Burki, P., Giuffredi, M. et al., Layer structured manganese oxide hydroxides IV. The buserite group: structure stabilization by transition elements, Chimia, 1975, 29: 517–520.
Burns, R. G., Burns, V. M., Stockman, H. W., A review of the to-dorokite-buserite problem: Implications to the mineralogy of marine manganese nodules, American Mineralogist, 1983, 68: 972–98.
Qian, J. C., Mellin, T. A., Study on exchange ability of metal ions in 1nm manganates, Acta Oceanologica Sinica (in Chinese with English abstracts), 1996, 18(4): 56–62.
Torgny, A., Mellin, Guobin, L., Stabilization of 10Å-manganates by interlayer cations and hydrothermal treatment: Implication for the mineralogy of marine manganese concretions, Chemical Geology, 1993, 115: 67–83.
Qian, J. C., Study on structural stability of 1nm manganates, Acta Oceanologica Sinica (in Chinese with English abstracts), 1998, 20(3): 56–63.
Luo, J., Zhang, Q. H., Aimin, H. et al., Double-aging method for preparation of stabilized Na-buserite and transformations to to-dorokites incorporated with various metals, Inorganic Chemistry, 1999, 38: 6106–6113.[DOI]
Shan, L. F., Yao, D., Mineral facies changes and secondary changes in ferro-manganese nodules, Acta Mineralogica Sinica (in Chinese with English abstracts), 1993, 13(2): 150–162.
Feng, X. H., Liu, F., Tan, W. F. et al., Synthesis of birnessite from the oxidation of Mn2+ by O2 in alkali medium: Effects of synthesis conditions, Clays and Clay Minerals, 2004, 52(2): 240–250.[DOI]
Kijima, N., Yasuda, H., Sato, T. et al., Preparation and characterization of open tunnel oxide α-MnO2 precipitated by ozone oxidation, Journal of Solid State Chemistry, 2001, 159(1): 94–102.[DOI]
Liu, E. H., Liu, P. A., Theory and Application of X-ray Diffraction Analyses (in Chinese), Beijing: Chemical Industry Press, 2003, 122–139.
Goff, P. L., Baffier, N., Bach, S. et al., Synthesis, ion exchange and electrochemical properties of lamellar phyllomangantates of the birnessite group, Materials Research Bulletin, 1996, 31(1): 63–75.[DOI]
Shen, Y. F., Suib, S. L., O’Young, C., Effects of inorganic cation templates on octahedral molecular sieves of manganese oxide, Journal of American Chemical Society, 1994, 116: 11020–11029.[DOI]
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, H., Feng, X., Liu, F. et al. Factors governing formation of todorokite at atmospheric pressure. Sci. China Ser. D-Earth Sci. 48, 1678–1689 (2005). https://doi.org/10.1360/01yd0550
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1360/01yd0550