Abstract
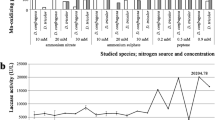
Lignocellulosic wastes such as neem hull, wheat bran, and sugarcane bagasse, available in abundance, are excellent substrates for the production of ligninolytic enzymes under solid-state fermentation by white-rot fungi. A ligninolytic enzyme system with high activity showing enhanced decomposition was obtained by cocultivation of Pleurotus ostreatus and Phanerochaete chrysosporium on combinations of lignocellulosic waste. Among the various substrate combinations examined, neem hull and wheat bran wastes gave the highest ligninolytic activity. A maximum production of laccase of 772 U/g and manganese peroxidase of 982 U/g was obtained on d 20 and lignin peroxidase of 656 U/g on d 25 at 28±1 °C under solid-state fermentation. All three enzymes thus obtained were partially purified by acetone fractionation and were exploited for decolorizing different types of acid and reactive dyes.
Similar content being viewed by others
References
Waldner, R., Leisola, M. S. A., and Fiechter, A. (1988), Appl. Microbiol. Biotechnol. 29, 400–407.
Evans, C. S. (1991), in Biodegradation: Natural and Synthetic Materials, Betts, W. B., ed., Springer Verlag, London, pp. 175–184.
Chen, H. C. A., Dostoretz, C. G., and Grethlein, H. E. (1991), Enzyme. Microb. Technol. 13, 404–407.
Hattaka, A. (1994), FEMS Microbiol. Rev. 13, 125–135.
De Jong, E., Field, J. A., and De Bont, J. A. M. (1992), FEBS Lett. 299, 107–110.
Buckley, K. F. and Dobson, A. D. W. (1998), Biotech. Lett. 20(3), 301–306.
Knapp, J. S., Newby, P. S., and Reece, L. P. (1994), Enzyme Microb. Technol. 17, 664–668.
Mehna, A., Bajpai, P., and Bajpai, P. K. (1993), Enzyme Microb. Technol. 17, 18–22.
Tatarko, M. and Bumpus, J. A. (1998), Water Sci. Technol. 32(5), 1713–1717.
Goering, H. K. and Van Soest, P. J. (1970), in Forage Fiber Analysis, Agricultural Handbook No. 379, Agricultural Research Service, Washington, DC, pp. 1–19.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951), J. Biol. Chem. 193, 265–275.
APHA. (1995), Standard methods for the production of water and wastewater, American Public Health Association, 19th ed., Washington, DC.
Palmieri, G., Giardina, P., Marzullo, L., Desiderio, B., Nitti, B., Cannio, R., and Sannia, G. (1993), Appl. Microbiol. Biotechnol. 39, 632–636.
Tien, M. and Kirk, T. K. (1988), Methods Enzymol. 161, 238–243.
Katagiri, N., Tsutsumi, Y., and Nishida, T. (1995), Appl. Environ. Microbiol. 61, 617–627.
Sasek, V., Volfova, O., Erbanova, P., Vyas, B. R. M., and Matucha, M. (1993), Biotechnol. Lett. 15, 521–526.
Kotterman, M. J. J., Rietberg, H. J., Hage, A., and Field, J. A. (1997), Biotechnol. Bioeng. 57, 220–227.
Schloser, D., Grey, R., and Fritsche, W. (1997), Appl. Microbiol. Biotechnol. 47, 412–418.
Huttermann, A., Geabauer, M., Volger, C., and Rosger, C. (1977), Holzforschung 31, 83–89.
Harrs, A., Chet, I., and Huttermann, A. (1981), Eur. J. Forest Pathol. 11, 67–76.
Ascher, K. R. S. (1993), Arch. Insect Biochem. Physiol. 22, 433–449.
Harrs, A. and Huttermann, A. (1983), Arch. Microbiol. 134, 1309–1313.
Sutherland, J. B., Pometto, A. L., and Crawford, D. L. (1983), Can. J. Bot. 61, 1194–1198.
Wong, Y. and Yu, J. (1999), Water Res. 33, 3512–3520.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, P., Madamwar, D. Production of ligninolytic enzymes for dye decolorization by cocultivation of white-rot fungi Pleurotus ostreatus and Phanerochaete chrysosporium under solid-state fermentation. Appl Biochem Biotechnol 102, 109–118 (2002). https://doi.org/10.1385/ABAB:102-103:1-6:109
Issue Date:
DOI: https://doi.org/10.1385/ABAB:102-103:1-6:109