Abstract
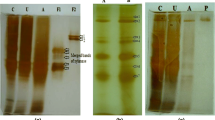
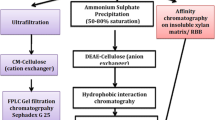
The alkalophilic bacteria Bacillus licheniformis 77-2 produces significant quantities of thermostable cellulase-free xylanases. The crude xylanase was purified to apparent homogeneity by gel filtration (G-75) and ionic exchange chromatography (carboxymethyl sephadex, Q sepharose, and Mono Q), resulting in the isolation of two xylanases. The molecular masses of the enzymes were estimated to be 17 kDa (X-I) and 40 kDa (X-II), as determined by SDS-PAGE. The K m and V max values were 1.8 mg/mL and 7.05 U/mg protein (X-I), and 1.05 mg/mL and 9.1 U/mg protein (X-II). The xylanases demonstrated optimum activity at pH 7.0 and 8.0–10.0 for xylanase X-I and X-II, respectively, and, retained more than 75% of hydrolytic activity up to pH 11.0. The purified enzymes were most active at 70 and 75°C for X-I and X-II, respectively, and, retained more than 90% of hydrolytic activity after 1 h of heating at 50°C and 60°C for X-I and X-II, respectively. The predominant products of xylan hydrolysates indicated that these enzymes were endoxylanases.
Similar content being viewed by others
References
Damiano, V. B., Bocchini, D. A., Gomes, E., and Da Silva, R. (2003), World J. Microbiol. Biotechnol., 19, 139–144.
Ohara, H. (2003), Appl. Microbiol. Biotechnol., 62, 474–477.
Mosier, N., Wyman, C., Dale, B., et al. (2005), Bioresour. Technol. 96, 673–686.
Alam, M., Gomes, I., Mohiuddin, G., and Hoq, M. M. (1994), Enzyme Microb. Technol. 16, 298–302.
Biely, P., Vrsanslá, M., and Kucar, S. (1992), In: Xylans and Xylamases, Visser, J., ed., Elsevier, Wageningen, The Netherlands, pp. 80–95.
Tavares, V. B., Gomes, E., and Da Silva, R. (1997), J. Brazilian Soc. Microbiol., 28, 179–182.
Archana, A. and Satyanarayana, T. (2003), World J. Microbiol. Biotechnol., 19, 53–57.
Miller, G. L. (1959), Anal. Chem., 31, 426–428.
Laemmli, U. K. (1970), Nature, 227, 680–685.
Meril, C. R. (1990), In: Methods in Enzymology, Deutscher, M. P. ed., Academic Press, New York, pp. 477–480.
McCroskery, P. A., Richards, J. F., and Harris, E. D. Jr. (1975), Biochem. J., 152, 131–142.
Breccia, J. D., Siñeriz, F., Baigorí, M. D., Castro, G. R., and Haiti-Kaul, R. (1998), Enzyme Microb. Technol. 22, 42–49.
Nakamura, S., Ishiguro, Y., Nakai, R., Wakabayashi, K., Aono, R., and Horikoshi, K. (1995), J. Mol. Cat. B. Enz., 1, 7–15.
Maiti, B. and Whitmire, D. (1997), Chem. Eng. Commun., 162, 169–175.
Beg, Q. K., Kapoor, M., Mahajan, L., and Hoondal, G. S. (2001), Appl. Microbiol. Biotechnol. 56, 326–338.
Khasin, A., Alchanati, I., and Shoham, Y. (1993), Appl. Environ. Microbiol. 59, 1725–1730.
Chen, C., Chen, J. L., and Lin, T. Y. (1997), Enzyme Microb. Technol., 21, 91–96.
Nath, D. and Rao, M. (2001), Enz. Microb. Technol., 28, 397–403.
Marques, S., Alves, L., Ribeiro, S., Girio, F. M., and Amaral Collaco, M. T. (1998) Appl. Biochem. Biotechnol. 73, 59–72.
Honda, H., Kudo, T., Ikura, Y., and Horikoshi, K. (1985), Canadian J. Microbiol., 31, 538–542.
Bastawde, K. B. (1992), World J. Microbiol. Biotechnol. 8, 353–368.
Devine, P. L., Warren, J. A., and Layton, G. T. (1990), Biotechniques, 8, 354–356.
Dixon, B. (1991), Biotechnology, 9, 418–418.
Christov, L. P. and Prior, B. A. (1993), Enzyme Microbiol. Technol. 15, 460–475.
Rudd, P. M., Joao, H. C., Coghill, E., et al. (1994), Biochemistry, 33, 17–22.
Wang, C., Eufemi, M., Turano, C., and Giartosio, A. (1996), Biochemistry, 35, 7299–7307.
Hamada, N., Ishikawa, K., and Fuse, N. J. (1999), Biosc. Bioeng. 87, 442–451.
Li, P., Gao, X. G. and Arellano, R. O. (2001), Protein Exp. Purification 22, 369–380.
Davis, B. G. (2002), Chem. Rev. 102, 579–601.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Damiano, V.B., Ward, R., Gomes, E. et al. Purification and characterization of two xylanases from alkalophilic and thermophilic Bacillus licheniformis 77-2. Appl Biochem Biotechnol 129, 289–302 (2006). https://doi.org/10.1385/ABAB:129:1:289
Issue Date:
DOI: https://doi.org/10.1385/ABAB:129:1:289