Abstract
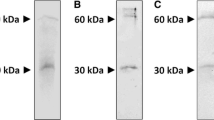
We have earlier shown that a unique membrane-bound enzyme mediates the transfer of acetyl group(s) from polyphenolic peracetates (PA) to functional proteins, which was termed acetoxy drug: protein transacetylase (TAase) because it acted upon several classes of PA. Here, we report the purification of TAase from human placentral microsomes to homogeneity with molecular mass of 60 kDa, exhibiting varying degrees of specificity to several classes of PA confirming the structure-activity relationship for the microsome-bound TAase. The TAase catalyzed protein acetylation by a model acetoxy drug, 7,8-diacetoxy-4-methyl coumarin (DAMC) was established by the demonstration of immunoreactivity of the acetylated target protein with anti-acetyl lysine antibody. TAase activity was severely inhibited in calcium-aggregated microsomes as well as when Ca2+ was added to purified TAase, suggesting that TAase could be a calcium binding protein. Furthermore, the N-terminal sequence analysis of purified TAase (EPAVYFKEQFLD) using Swiss Prot Database perfectly matched with calreticulin (CRT), a major microsomal calcium binding protein of the endoplasmic reticulum (ER). The identity of TAase with CRT was substantiated by the observation that the purified TAase avidly reacted with commercially available antibody raised against the C-terminus of human CRT (13 residues peptide, DEEDATGQAKDEL). Purified TAase also showed Ca2+ binding and acted as a substrate for phosphorylation catalyzed by protein kinase C (PKC), which are hallmark characteristics of CRT. Further, purified placental CRT as well as the commercially procured pure CRT yielded significant TAase catalytic activity and were also found effective in mediating the acetylation of the target protein NADPH cytochrome P-450 reductase by DAMC as detected by Western blot using anti-acetyl lysine antibody. These observations for the first time convincingly attribute the transacetylase function to CRT. Hence, this transacetylase function of CRT is designated calreticulin transacetylase (CRTAase). We envisage that CRTAase plays an important role in protein modification by way of acetylation independent of Acetyl CoA.
Similar content being viewed by others
References
Allis, C. D., Chicoine, L. G., Richman, R., and Schulman, G. (1985) Deposition-related histone acetylation in micronuclei of conjugating tetrahymena. Proc. Natl. Acad. Sci. USA 82, 8048–8052.
Vane, J. R. (1971) Inhibition of prostaglandin synthesis as a mechanism of action for aspirin like drugs. Nature (New Biol) 231, 232–234.
Lee, T. C., Uemura, Y., and Snyder, F. (1992) A novel CoA-independent transacetylase produces the ethanolamine plasmalogen and acyl analogs of platelet-activating factor (PAF) with PAF as the acetate donor in HL-60 cells. J. Biol. Chem. 267, 19,992–20,001.
Weber, W. W. and Hein, D. W. (1985) N-acetylation pharmacogenetics. Pharmacol. Rev. 37, 25–79.
Raj, H. G., Parmar, V. S., Jain, S. C., et al. (1998) Mechanism of biochemical action of substituted 4-methylbenzopyran-2-one. Part II: mechanism-based inhibition of rat liver microsome-mediated aflatoxin B1-DNA binding by the candidate antimutagen 7,8-diacetoxy-4-methylcoumarin. Bioorg. Med. Chem. 6, 1895–1904.
Raj, H. G., Parmar, V. S., Jain, S. C., et al. (1999) Mechanism of biochemical action of substituted 4-methylbenzopyran-2-ones. Part 4: hyperbolic activation of rat liver microsomal NADPH-cytochrome c reductase by the novel acetylator 7,8-diacetoxy-4-methylcoumarin. Bioorg. Med. Chem. 7, 369–373.
Raj, H. G., Parmar, V. S., Jain, S. C., et al. (2000) Mechanism of biochemical action of substituted 4-methylbenzopyran-2-ones. Part 7: assay and characterization of 7,8-diacetoxy-4-methylcoumarin: protein transacetylase from rat liver microsomes based on the irreversible inhibition of cytosolic glutathione S-transferase. Bioorg. Med. Chem. 8, 1707–1712.
Kohli, E., Gaspari, M., Raj, H. G., et al. (2002) Establishment of the enzymatic protein acetylation independent of acetyl CoA: Recombinant glutathione S- transferase 3-3 is acetylated by a novel membrane-bound transacetylase using 7,8-diacetoxy-4-methyl coumarin as the acetyl donor. FEBS Lett. 530, 139–142.
Kohli, E., Gaspari, M., Raj, H. G., et al. (2004) Acetoxy drug: protein transacetylase of buffalo liver-characterization and mass spectrometry of the acetylated protein product. Biochim. Biophys. Acta. 1698, 55–66.
Raj, H. G., Singh, B. K., Kohli, E., et al. (2005) Acetoxy drug: protein transacetylase: A novel enzyme-mediating protein acetylation by polyphenolic peracetates. Pure Appl. Chem. 77, 245–250.
Raj, H. G., Kohli, E., Rohil, V., et al. (2001) Acetoxy-4-methylcoumarins confer differential protection from aflatoxin B1 induced micronuclei and apoptosis in lung and bone marrow cells. Mutation Res. 494, 31–40.
Raj, H. G., Malik, S., Parmar, V. S., et al. (2001) Chemoprevention of benzene induced bone marrow and pulmonary genotoxicity. Teratogen Carcin. Mut. 21, 181–187.
Khurana, P., Kumari, R., Vohra, P., et al. (2005) Acetoxy Drug: Protein Transacetylase catalyzed activation of human platelet nitric oxide synthase by polyphenolic acetates. Bioorg. Med. Chem. 14, 575–583.
Kumari, R., Kohli, E., Gupta, G., et al. (2002) Microsomal acetoxy drug: protein transacetylase of placenta: part 1. Characterization of DAMC: GST transacetylase. Placenta 23, 352–357.
Lowry, O. H., Rosebrough, N. J., Farr, A. T., and Randall, R. J. (1951) Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275.
Habig, W. H., Pabst, M. J., and Jacoby, W. B. (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139.
Dey, A. C., Rahal, S., Rimsay, R. L., and Senciall, I. R. (1981) A simple procedure for the solubilization of NADH-cytochrome b5 reductase. Anal. Biochem. 110, 373–379.
Ornstein, L. (1964) Disc electrophoresis-I. Background and theory. Ann. NY Acad. Sci. 121, 404–427.
Davis, B. J. (1964) Disc electrophoresis-II. Method and application to human serum proteins. Ann. NY Acad. Sci. 121, 404–427.
Laemmli, U. K. (1970) Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature (London) 227, 680–685.
Ferreras, M. and Jose, G. (1993) Zn+2 reverse staining: A method for detection and quantification of protein in PAGE. Anal. Biochem. 213, 206–212.
Weber, K. and Osborn, M. (1969) The reliability of molecular weight determination by Dodecyl sulfate- polyacrylamide gel electrophoresis. J. Biol. Chem. 244(16), 4406–4412.
Boyum, A. (1976) Isolation of lymphocytes, granulocytes and macrophages. Scand. J. Immunol. Suppl. 5, 9–15.
Bansal, S. K., Jha, A., and Jaiswal, A. S. (1997) Increased levels of protein kinase c in lymphocytes in asthmas: possible mechanism of regulation. Eur. Respir. J. 10, 308–313.
Li, Z. and Komatsu, S. (2000) Molecular cloning and characterization of calreticulin, a calcium-binding protein involved in the regeneration of rice-cultured suspension cells. Eur. J. Biochem. 267, 737–745.
Singh, I., Kohli, E., Raj, H. G., et al. (2002) Mechanism of Biochemical action Substituted-4-methyl Benzopyran-2-ones. Part 9: comparison of acetoxy-4-methyl coumarins and other polyphenolic acetates reveal the specificity acetory: drug protein transacetylase for pyran carbonyl group in proximity to the oxygen heteroatom. Bioorg. Med. Chem. 10, 4103–4111.
Raj, H. G., Parmar, V. S., Tyagi, Y. K., et al. (2001) Mechanism of biochemical action of substituted Benzopyran-2-ones, part 8: acetoxycoumarin: protein transacetylase-specificity for aromatic nuclear acetoxy groups in proximity to the oxygen heteroatom. Bioorg. Med. Chem. 9, 1085–1089.
Kamath, S. A. and Rubin, F. (1972) Interaction of calcium with microsomes: a modified method for the rapid isolation of rat liver microscomes. Biochem. Biophys. Res. Commun. 49(1), 52–59.
Treveso, S., Zorzato, F., Chiozzi, P., Melandri, P., Volpe, P., and Pozzen, T. (1991) Frog brain expresses a 60kDa Ca2+ binding protein similar to mammalian calreticulin. Biochem. Biophys. Res. Commun. 175(2), 444–450.
Hassan, A. M., Wesson, C., and Trumble, W. R. (1995) Calreticulin is the major Ca2+ storage protein in the endoplasmic reticulum of the pea plant (Pisum sativum). Biochem. Biophys. Res. Commun. 211(1), 54–59.
Milner, R. E., Baksh, S., Shemanko, G., et al. (1991) Calreticulin, and not calsequestrin, is the major calcium binding protein of smooth muscle sarcoplasmic reticulum and liver endoplasmic reticulum. J. Biol. Chem. 266(11), 7155–7165.
Denning, G. M., Leidal, K. G., Holst, V. A., et al. (1997) Calreticulin biosynthesis and processing in human myeloid cells: demonstration of signal peptide cleavage and N glycosylation. Blood 90(1), 372–381.
Houen, G. and Koch, C. (1994) Human placental calreticulin: purification, characterization and association with other proteins. Acta Chem. Scand. 48(11), 905–911.
Hojrup, P., Roepstorff, P., and Houen, G. (2001) Human placental calreticulin: characterization of domain structure and posttranslational modifications. Eur. J. Biochem. 268, 2558–2565.
Michalak, M., Corbett, E. F., Mesaeli, N., Nakamura, K., and Opas, M. (1999) Calreticulin: one protein, one gene, and many function. Biochem. J. 344, 281–292.
Rendon-Huerta, E., Mendoza-Hernandez, G., and Robles-Flores, M. (1999) Characterization of calreticulin as a protein interacting with protein kinase c. Biochem. J. 344, 469–475.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seema, Kumari, R., Gupta, G. et al. Characterization of protein transacetylase from human placenta as a signaling molecule calreticulin using polyphenolic peracetates as the acetyl group donors. Cell Biochem Biophys 47, 53–64 (2007). https://doi.org/10.1385/CBB:47:1:53
Issue Date:
DOI: https://doi.org/10.1385/CBB:47:1:53