Abstract
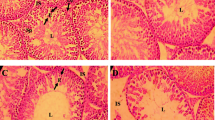
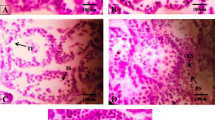
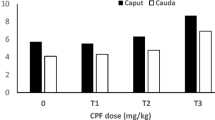
It has been proposed that a global decline in sperm counts, semen quality, and several male reproductive disorders are associated with exposure to environmental chemicals. Thus, the present study examined the effects of two estrogenic chemicals, octylphenol (OP) and bisphenol A (BPA), on epididymal sperm counts and sperm motility, luteinizing hormone (LH)-releasing hormone (LHRH)-stimulated plasma LH and steroid hormones, insulin-like growth factor I (IGF-I), and accessory reproductive organs in pubertal male Wistar rats. Fifty-day-old rats in the OP group (n=11) and BPA group (n=11) received daily sc injections of the respective chemical at a dose of 3 mg/kg bw dissolved in 0.2 mL DMSO. Rats in the control group (DMSO group; n=10) received 0.2 mL DMSO alone. After 2 wk of treatment, a jugular blood sample was taken, and, on the next day, a second blood sample was taken 1 h after an sc injection of LHRH (250 ng). After 5 wk of treatment, rats were deeply anesthetized and heart blood was collected. Epididymal sperm motility and sperm head counts were determined. LHRH increased plasma LH to higher levels in all groups, but the increases were significant (p<0.01) in the BPA and OP groups. However, despite higher LH levels after LHRH injection, the incremental responses of testosterone and progesterone in the OP and BPA groups were small compared to those in the DMSO group, which showed a small LH response. After 5 wk of treatment, plasma testosterone levels were significantly (p<0.01) reduced in the OP and BPA groups and this was accompanied by reduced (p<0.05) epididymal sperm counts. However, the chemical-treated groups had high basal progesterone levels. No significant effects of chemicals on sperm motility parameters were noted. The chemical-induced increases (p<0.05) of the weight of ventral prostate gland were coincided with elevated plasma IGF-I levels in the BPA (p<0.05) and OP (p<0.01) groups. The present results demonstrated that OP and BPA can reduce sperm counts resulting from lowered plasma testosterone in male rats just after puberty. The enlarged ventral prostate gland may possibly be associated with increased plasma IGF-I, raising the possibility of a link between these chemicals and prostate diseases because IGF-I has been implicated in the pathogenesis of human prostate cancers.
Similar content being viewed by others
References
Sharpe, R. M., Fisher, J. S., Millar, M. M., Joblin, S., and Sumpter, J. P. (1995). Environ. Health Perspect. 103, 1136–1143.
Toppari, J., Larsen, J. C., and Christiansen, P., et al. (1996). Environ. Health Perspect. 104(Suppl 4), 741–803.
Krishnan, A. V., Stathis, P., Permuth, S. F., Tokes, L., and Feldman, D. (1993). Endocrinology 132, 2279–2286.
White, R., Joblin, S., Hoare, S. A., Sumpter, J. P., and Parker, M. G. (1994). Endocrinology 135, 175–182.
Steinmetz, R., Brown, N. G., Allen, D. L., Bigsby, R. M., and Ben-Jonathan, N. (1997). Endocrinology 138, 1780–1786.
vom Saal, F. S., Cooke, P. S., Buchanan, D. L., et al. (1998). Toxicol. Ind. Health 14, 239–260.
Fisher, J. S., Turner, K. J., Brown, D., and Sharpe, R. M. (1999). Environ. Health Perspect. 107, 397–405.
Herath, C. B., Watanabe, G., Katsuda, S., Yoshida, M., Suzuki, A. K., and Taya, K. (2001). Biol. Reprod. 64, 1216–1224.
Giger, W., Brunner, P. H., and Schaffner, C. (1984). Science 225, 623–625.
Ben-Jonathan, N. and Steinmetz, R. (1998). Trends Endocrinol. Metabol. 9, 124–128.
Olea, N., Pulgar, R., Perez, P., et al. (1996). Environ. Health Perspect. 104, 298–305.
Schonfelder, G., Wittfoht, W., Hopp, H., Talsness, C. E., Paul, M., and Chahoud, I. (2002). Environ. Health Perspect. 110, A703-A707.
Boockfor, F. R. and Blake, C. A. (1997). Biol. Reprod. 57, 267–277.
Lee, P. C. (1998). Endocrine 9, 105–111.
Lee, P. C., Arndt, P., and Nickels, K. C. (1999). Endocrine 11, 61–68.
Plonowski, A., Schally, A. V., Varga, J. L., et al. (2000). Prostate 44, 172–180.
Tohei, A., Suda, S., Taya, K., Hashimoto, T., and Kogo, H. (2001). Exp. Biol. Med. 226, 216–221.
Saunders, P. T., Majdic, G., Parte, P., et al. (1997). Adv. Exp. Med. Biol. 424, 99–110.
Takao, T., Nanamiya, W., Nagano, I., Asaba, K., Kawabata, K., and Hashimoto, K. (1999). Life Sci. 65, 2351–2357.
Fukuda, M., Miyamoto, K., Hasegawa, Y., Ibuki, Y., and Igarashi, M. (1987). Mol. Cell. Endocrinol. 51, 41–50.
Kotsuji, F., Winters, S. J., Keeping, H. S., Attardi, B., Oshima, H., and Troen, P. (1988). Endocrinology 122, 2796–2802.
Farnworth, P. G., Robertson, D. M., de Kretser, D. M., and Burger, H. G. (1988). J. Endocrinol. 119, 233–241.
Wang, Q. F., Farnworth, P. G., Findlay, J. K., and Burger, H. G. (1989). Endocrinology 124, 363–368.
Herath, C. B., Watanabe, G., Wanzhu, J., et al. (2001). J. Androl. 22, 838–846.
Arai, K., Watanabe, G., Taya, K., and Sasamoto, S. (1996). Biol. Reprod. 55, 127–133.
Ewing, L. L., Wing, T. Y., Cochran, R. C., Kromann, N., and Zirkin, B. R. (1983). Endocrinology 112, 1763–1769.
Valladares, L. E., Ronco, A. M., and Pino, A. M. (1986). J. Endocrinol. 110, 551–556.
Murono, E. P., Derk, R. C., and de Leon, J. H. (1999). Reprod. Toxicol. 13, 451–462.
Murono, E. P., Derk, R. C., and de Leon, J. H. (2000). Reprod. Toxicol. 14, 275–288.
Murono, E. P. and Derk, R. C. (2002). J. Steroid. Biochem. Mol. Biol. 81, 181–189.
Nikula, H., Talonpoika, T., Kaleva, M., and Toppari, J. (1999). Toxicol. Appl. Pharmacol. 157, 166–173.
Song, K. H., Lee, K., and Choi, H. S. (2002). Endocrinology 143, 2208–2215.
Nejaty, H., Lacey, M., and Whitehead, S. A. (2001). Exp. Biol. Med. 226, 570–576.
Al-Hiyasat, A. S., Darmani, H., and Elbetieha, A. M. (2002). Eur. J. Oral Sci. 110, 163–167.
Raychoudhury, S. S., Blake, C. A., and Millette, C. F. (1999). Toxicol. Appl. Pharmacol. 157, 192–202.
Chitra, K. C., Latchoumycandane, C., and Mathur, P. P. (2003). Toxicology 185, 119–127.
Takahashi, O. and Oishi, S. (2003). Food Chem. Toxicol. 41, 1035–1044.
Nagel, S. C., vom Saal, F. S., Thayer, K. A., Dhar, M. G., Boechler, M., and Welshons, W. V. (1997). Environ. Health Perspect. 105, 70–76.
Gupta, C. (2000). Proc. Soc. Exp. Biol. Med. 224, 61–68.
vom Saal, F. S., Timms, B. G., Montano, M. M., et al. (1997). Proc. Natl. Acad. Sci. USA 94, 2056–2061.
Gupta, C. (2000). Urol. Res. 28, 223–229.
Khurana, S., Ranmal, S., and Ben-Jonathan, N. (2000). Endocrinology 141, 4512–4517.
Stoker, T. E., Robinette, C. L., Britt, B. H., Laws, S. C., and Cooper, R. L. (1999). Biol. Reprod. 61, 1636–1643.
Sahlin, L., Norstedt, G., and Eriksson, H. (1994). J. Steroid. Biochem. Mol. Biol. 51, 57–66.
Johnson, B. J., White, M. E., Hathaway, M. R., Christians, C. J., and Dayton, W. R. (1998). J. Anim. Sci. 76, 491–497.
Campagnoli, C., Biglia, N., Cantamessa, C., Lesca, L., Lotano, M. R., and Sismondi, P. (1998). Gynecol. Endocrinol. 12, 259–266.
Huynh, H. T., Tetenes, E., Wallace, L., and Pollak, M. (1993). Cancer Res. 53, 1727–1730.
Metzger, D. L. and Kerrigan, J. R. (1994). J. Clin. Endocrinol. Metab. 79, 513–518.
Klotz, D. M., Hewitt, S. C., Korach, K. S., and Diaugustine, R. P. (2000). Endocrinology 141, 3430–3439.
Chan, J. M., Stampfer, M. J., Giovannucci, E., et al. (1998). Science 279, 563–566.
Shiraishi, H., Carter, D. S., and Hites, R. A. (1989). Biomed. Environ. Mass Spectrom. 18, 478–483.
Clark, L. B., Rosen R. T., Hartman, T. G., et al. (1992). Int. J. Environ. Anal. Chem. 47, 167–180.
Tohei, A., Tomabechi, T., Mamada, M., Akai, M., Watanabe, G., and Taya, K. (1997). J. Vet. Med. Sci. 59, 329–334.
Taya, K., Watanabe, G., and Sasamoto, S. (1985). Jpn. J. Ani. Reprod. 31, 186–197.
Blum, W. F. and Breier, B. H. (1994). Growth Regul. 4(Suppl 1), 11–19.
Hamada, T., Watanabe, G., Kokuho, T., et al. (1989). J. Endocrinol. 122, 697–704.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herath, C.B., Jin, W., Watanabe, G. et al. Adverse effects of environmental toxicants, octylphenol and bisphenol A, on male reproductive functions in pubertal rats. Endocr 25, 163–172 (2004). https://doi.org/10.1385/ENDO:25:2:163
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/ENDO:25:2:163