Abstract
Objective
This study aimed to synthesize available evidence on the effectiveness and safety of COVID-19 vaccines for frail older adults through a rapid review, supplemented with geriatricians’ consensus statements.
Methods
References were identified through MEDLINE and Web of Science on 1st February 2021 using relevant terms related to COVID-19, vaccine, and older adults. Searches were also conducted on reference lists of review articles and Google Scholar. The content was updated on 8th April via hand searching. We included studies on Phase III randomized controlled trials, and data from real world administration of vaccines. A two-round Delphi study was conducted with 15 geriatricians to elicit their thoughts and recommendations regarding COVID-19 vaccination for frail older adults.
Results
Five Phase III randomized controlled efficacy trials reported vaccine efficacy ranging from 66.7% to 95% among participants aged 16 to 95. The vaccine efficacy for participants aged 65 and above is 94.7% and 86.4% for Pfizer-BioNTech and Moderna respectively. Sputnik V reported a vaccine efficacy of 91.8% for participants 60 and above. Serious adverse events were reported by 0.27% to 1% of participants who received at least one dose of the four vaccines. For the Delphi study, 16 out of 24 statements achieved consensus. The Delphi panel opined that frail or very old adults, except those with limited life expectancy, should be vaccinated due to their vulnerability. They also agree that vaccination decisions should be made by patients when possible, with the involvement of next-of-kin should the frail older adult be unable to do so. Lastly, the panel thought that frail older adults should be included in future clinical trials.
Conclusion
In early clinical trials, there is paucity of evidence on efficacy and safety of current COVID-19 vaccines among frail older adults. Geriatricians’ consensus indicate that frail older adults should be vaccinated except where life expectancy is limited. Future trials assessing efficacy and safety should include frail older adults.
Similar content being viewed by others
Introduction
The emergence of COVID-19 had a devastating impact, with over 132 million confirmed cases and 2.8 million deaths worldwide as of 7th of April 2021 (1). Frailty has been found to be associated with an increase in severity and risk of adverse events from COVID-19 (2, 3). In Singapore, one in six citizens is 65 and above (4), and 5.7% of older adults aged 60 and older are frail (5), which translates to an estimated 35,000 frail older adults in Singapore. Similar to other countries with high older adult population, this creates an urgency to vaccinate older adults as soon as possible.
After early data from Phase III trials were published in 2020, both COVID-19 vaccines Pfizer-BioNTech and Moderna were given emergency approval to curb the raging pandemic (6, 7). Though trial results are promising, efficacy and safety of these vaccines on frail older adults is largely unknown. These studies were conducted in a controlled clinical environment on relatively healthy individuals with strict inclusion and exclusion criteria (8, 9). Albeit this mass vaccination program of older adults is currently underway worldwide. However, with some emerging reports on the deaths of frail older adults after vaccination, questions have been raised regarding the safety (10), efficacy, ethical issues surrounding informed consent (11), and whether frail older adults should be vaccinated given the risks.
Thus, the aim of this study is to provide insights into the question: Are COVID-19 vaccines effective and safe for frail older adults? Due to the urgent nature of the situation, we chose to conduct a rapid review and to supplement our findings with a Delphi study among geriatricians on their views on a) current evidence of efficacy and safety of COVID-19 vaccination for frail older adults, b) recommendations on vaccinating older adults, and c) ethical issues around it.
Methods
Rapid Review
A literature search was conducted on 1st of February 2021 in Medline and Web of Science using a search strategy formulated with the PICOTS framework (population, interest, comparison, outcomes, timeframe, settings). Table 1 shows keywords adopted for the search. Appendix 1 shows the full search strategy in Medline.
In addition, we also searched Google Scholar using simplified key words “COVID-19 vaccination(s)” and “older adults” to identify grey literature, and searched the reference list of discussion articles relating to COVID-19 vaccines. The title and abstract of these articles were then subjected to an initial screening process against a set of inclusion and exclusion criteria by two reviewers (JG, PL), followed by full-text screening (see Appendix 2 for full eligibility criteria).
The screening process was conducted by the reviewers independently in Covidence, a web-based tool (12). In both screening processes, any disagreements were resolved via discussion with a third reviewer (PPG). The following data variables were extracted using Covidence: basic publication information, information on the study type and design, sample, vaccine efficacy, and safety (e.g. side effects, adverse effects) and subsequently exported into a spreadsheet. Meta-analysis was not possible as the studies had clinical and methodological heterogeneity, Clinical heterogeneity was evident in the studies for defining COVID-19 symptoms. For example, the studies differed in their definition of fever as part of a COVID-19 diagnosis (e.g. AstraZeneca defined it as ≥37.8, Moderna as ≥38, Sputnik V as ≥ 38.5), and number of symptoms (e.g. AstraZeneca requires one symptom from a list while Pfizer and Moderna requires two). Also, methodological heterogeneity was evident across the studies as vaccine efficacy was measured at different time points for the available vaccines. Pfizer’s efficacy outcome was a confirmed COVID diagnosis 7 days after the second dose, Sputnik V was 21 days after the first dose, and Moderna’s was 14 days after the second dose.
Delphi Study
The Delphi technique is commonly used to gather informed consensus on a topic that does not have clear evidence (14). An online Delphi study was used to solicit the opinions of geriatricians in Singapore on recommendations regarding COVID-19 vaccination for frail older adults. As in the case of COVID-19 vaccines, where real world data is still limited, the available evidence to date would be supplemented with the clinical expertise of geriatricians to arrive at consensus statements for guideline development.
Formulation of Statements
Statements for the survey were first drafted by the research team based on suggestions from a geriatrician and by adapting statements from other Delphi studies on vaccines (15–17). This was followed by a presentation of the rapid review results to the Chapter of Geriatricians COVID-19 Vaccination Workgroup in Singapore, where they gave their input for the statements as well. One of the geriatricians provided further advice regarding statements on ethics. Twenty Delphi statements were formulated for round 1 of the study. These statements were divided into three categories: (a) overall literature on COVID-19 vaccination for frail older adults, (b) clinical opinion and experience regarding COVID-19 vaccination for frail older adults, and (c) ethical issues surrounding vaccinating frail older adults.
Delphi Panel
In Delphi exercises, a minimum of 12 respondents is generally considered to be sufficient to enable consensus to be achieved, larger sample sizes can provide diminishing returns regarding the validity of the findings (18). Nevertheless, Delphi sample sizes are dependent on group dynamics in reaching consensus rather than their statistical power (19). As we were targeting a homogeneous expert group with similar trainings and experiences, a non-probability purposive sample of 20 geriatricians were invited via email to participate in this Delphi study. The geriatricians invited were consultants, senior consultants, or heads of Geriatric Medicine departments of public hospitals in Singapore. One of them also heads a research institute focusing on geriatric research in Singapore.
Delphi Process and Consensus
The panelists were asked to rate the statements on a scale of 1 (strongly disagree) to 7 (strongly agree). An a priori criteria was used to define consensus, based on the level of agreement or disagreement, and on the dispersion of responses using the width of interquartile range (20, 21). Consensus is deemed to have been achieved if 75% of respondents disagreed (defined as a rating of 1 and 2) or agreed (defined as a rating of 6 and 7) with a statement, and if the interquartile range of the response is 1 or less. Round 2 of the study contained statements that did not reach consensus in round 1, with some refinements and additional statements based on the comments provided by the panelists. Both rounds of the study were conducted via email. The survey statements for round 1 and round 2 can be found in Appendix 3.
To complete the Delphi process, participants were required to respond to both rounds. Those who did not respond to Round 1 were not invited to Round 2. A dropout rate of 20% was expected over the two rounds, in accordance with previous Delphi studies (22, 23). We aimed to recruit and complete the process with at least 12 geriatricians.
Results
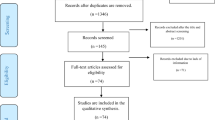
A total of 697 articles were identified using the initial search. After applying the eligibility criteria to the initial screening and full-text screening, five articles were included for the review (8, 9, 24–26). Additionally, one article was recommended by a panelist during the Delphi process (27), while another four articles were found during the update search in Google Scholar using the same search strategy on 8th of April (28–31). Figure 1 shows the PRISMA flow diagram of the whole process (32). Among the included studies, majority of them were peer-reviewed while a few were pre-prints (see Appendix 4).
COVID-19 Vaccines
Three vaccines (Pfizer-BioNTech, Moderna, and Sputnik V) had published data on completed Phase III studies (8, 9, 24) while one vaccine (AstraZeneca) had published two sets of data, one from their UK trial site, and the other from an interim analysis of Phase I/II/III studies from four trial sites (25, 26). Some countries that have begun administering Pfizer-BioNTech and/or AstraZeneca have published their data as well (27–29).
Vaccine Efficacy and Adverse Effects
Studies on the four vaccines included participants aged 16 to 95 years (see Appendix 7 for a breakdown of older adults involved in each trial), and reported efficacy ranging from 66.7–95% (Table 2) (8, 9, 24–26). The vaccine efficacy (see Appendix 6 for a summary of how vaccine efficacy is assessed) for participants aged 65 and above is 94.7% and 86.4% for Pfizer-BioNTech and Moderna respectively. Sputnik V reported a vaccine efficacy of 91.8% for participants aged 60 and above. At that point in time, vaccine efficacy by age was not available for AstraZeneca as they combined Phase II/III clinical trials and were measuring participants’ immunogenicity. Common adverse effects reported across the four vaccines were fatigue, muscle aches, headaches, and chills. Serious adverse events such as abdominal pain, angina pectoris, atrial fibrillation, and myocardial infarction were reported by 0.27–1% of participants who received at least one dose of the four vaccines (8, 9, 24–26).
Vaccine Effectiveness/Antibody Response
Researchers in England reported a vaccine effectiveness of 70% among those 80 years and above 28–34 days after receiving their first dose of Pfizer-BioNTech vaccine, with the effectiveness increasing to 89% 14 days after receiving the second dose (28). This cohort received priority vaccination before 4th of January 2021. For those receiving their vaccination after this date, where the data is only for one dose, vaccine effectiveness among those 70 years or older was found to be at 59% 28–34 days post first dose for Pfizer BioNTech vaccine and at 60%, 28–34 days for post first dose of AstraZeneca vaccine. However, at 35+ days post vaccination (dose 1), the vaccine effectiveness dropped slightly to 57% for Pfizer BioNTech vaccine, while it increased to 73% for the AstraZeneca vaccine.
In Scotland, researchers reported a vaccine effectiveness of 81% for those 80 and above and 79% for those between 65 and 79 years old at 28 to 34 days after one dose of either the Pfizer BioNTech or AstraZeneca vaccine (29). The vaccine effectiveness was assessed against hospitalization within 28 days of being tested positive for COVID-19. Of note, the vaccine effectiveness for those between 65 and 79 years decreased to 56% after 35 days of receiving one dose of either the Pfizer-BioNTech or AstraZeneca vaccine. In Israel, Pfizer-BioNTech vaccine has an overall vaccine effectiveness of 60% for documented infection, 66% for symptomatic Covid-19, and 78% for hospitalization within 21–27 days of receiving the first dose, and the respective effectiveness increased to 92%, 94%, and 87% after 7 days of the second dose (27). Among those who were 70 and older, vaccine effectiveness was 50% for documented infection and 64% for symptomatic illness 21 to 27 days after the first dose, rising to 95% and 98% respectively after 7 days of the second dose.
Additionally, a study on a group (n=100) of older adults (80–96 years) indicated that two doses of Pfizer-BioNTech vaccine elicited a strong humoral immunity and retained broad efficacy against the Brazilian variant of COVID-19 (31). In another longitudinal study of 134 long-term care facilities residents in Spain, where 44% of residents were classified as frail, two doses of Pfizer-BioNTech vaccine elicited antibody response at 21.9 days (average), suggesting antibody response regardless of frailty, disability, older age, sex, cognitive impairment, or comorbidities (30).
Delphi Study
The response rates for Delphi survey round one and two were 75% (15/20) and 100% (15/15) respectively. A total of 16 statements achieved consensus from both rounds, while eight did not (see Figure 2 for an overview by category). The 16 consensus statements and their percentages of agreement can be found in Table 3. From Figure 2, the category with the least consensus was on statements based on clinical opinion and experience. A list of the remaining statements that did not achieve consensus can be found in Appendix 5, while the full list of statements can be found in Appendix 3.
While the consensus is that there is no evidence to suggest COVID-19 vaccination for older adults is less effective or less safe compared to the general population, a panelist acknowledged the need to further investigate reports of deaths of frail nursing home residents in Europe after vaccination (10). Additional comments by panelists included the need for an individualized risk benefit assessment when recommending the vaccine to frail older adults, and that the prioritization of frail older adults for vaccination should also consider the probability of contracting COVID-19. In countries where COVID-19 is raging, there is a clear benefit to prioritizing frail older adults. However, for countries with no community spread, the benefits may not be as clear and may require decisions to be made on a case-by-case basis.
Discussion
To the best of our knowledge, this is the first rapid review to summarize evidence on efficacy and safety of COVID-19 vaccines along with consensus statements from geriatricians based on current evidence.
COVID-19 Vaccination for Frail Older Adults — Clinical Trial and Real-World Evidence
Though results from Phase III trials showed high efficacy and low adverse events among the general population, there is a paucity of information about the effects of these vaccines on frail older adults (8, 9, 24–26). Despite the inclusion of older adults, studies either did not provide sufficient information to determine the frailty status of participants or had strict inclusion and exclusion criteria that were likely to exclude frail older adults in their sample. For example, AstraZeneca’s clinical trial excluded participants that had a Clinical Frailty Score of 4 or higher (33). Participants included in the trials for three vaccines (Pfizer-BioNTech, Moderna, and Sputnik V) were generally healthy or had chronic comorbidities such as diabetes, obesity, or heart disease (8, 9, 24). However, the proportion of older adults with these health issues are not known. Additionally, frailty indicators such as: (a) level of physical activity, (b) limitations to activities of daily living, (c) walking speed, or (d) loss of weight were not reported (34–36). Hence, we were unable to determine if frail older adults were included in these trials. The AstraZeneca study combined information from different trials worldwide, with each trial having slightly different inclusion criteria (25, 26). Generally, participants (aside from some diagnosed with HIV) were healthy high-risk adults (e.g. healthcare workers). Based on the information reported in these studies, vaccine efficacy among frail older adults is unknown.
Similarly, real world studies of vaccine effectiveness reported promising results, but there is insufficient information on frail older adults (27–29). All three countries with published data on their vaccination program had large samples of older adults, and reported positive results such as lower hospitalization rates and deaths for those who were vaccinated. However, the proportion of frail older adults in these large samples is generally unknown. Like other vaccine studies where older adults tend to be not well studied (37), there is considerable lack of frail older adults’ representations in COVID-19 vaccine trials.
To supplement the lack of large data, information from smaller serological studies that focused on only older adults could be helpful. For example, a study on older adults (≥ 80 years) in a hospital in England reported an adjusted vaccine effectiveness of 71.4% and 80.4% 14 days after one dose of Pfizer BioNTech or AstraZeneca respectively (38). Of note, 85% of older adults in this study scored between 5–9 on the Rockwood Clinical Frailty Scale. On the other hand, heterogeneity of immune responses has been observed in older populations due to their waning immune response, with one study reporting 31.3% of those above 80 having no detectable antibodies despite receiving two doses of Pfizer BioNTech (39). However, evidence from two other studies demonstrated that two doses of Pfizer-BioNTech’s vaccine produces immunogenicity, independent of older adults’ health conditions (30), and provided strong humoral immunity in older adults from 80 to 96 years of age (31).
Delphi Study
Overall, the geriatricians agreed that there is currently no evidence indicating that COVID-19 vaccines would be less effective and safe for older adults, as well as on the ethical issues surrounding decision-making for vaccinating frail older adults. However, they were unable to agree on whether current evidence is sufficient to guide vaccine recommendations for both older adults and frail older adults. While they were in favor of frail older adults being vaccinated, the geriatricians advised against vaccinating frail older adults with less than six months life expectancy. They also conflicted over statements concerning whether certain subgroups of frail older adults would benefit from the vaccination.
Lastly, the geriatricians indicated that frail older adults should be included in future clinical trials of the vaccine. Other geriatricians have also argued that clinical trials of vaccines have not included frail older adults who are most in need of the protection (40), raising questions about the safety and efficacy of these vaccines, and whether they are optimized for frail older adults who might respond differently compared to younger adults. The panelists’ responses were consistent with our findings that there is limited evidence one can draw upon to make a recommendation on vaccinating frail older adults. Although the Delphi method has its limitations and not comparable to evidence-based methods, it is used in health sciences when there is a lack of evidence from randomized control trials due to a new phenomenon or exclusion of certain population in trials as a result of ethical or pragmatic reasons (14). As COVID-19 is a new phenomenon with limited evidence, geriatricians are the source of information that others might refer to when treating older adults. Hence, the collective statements would serve as a guide in terms of clinical considerations.
In sum, data from Phase III clinical trials provided limited evidence on the effects of the four vaccines on frail older adults. However, emerging real-world evidence shows benefits of vaccination for Pfizer-BioNTech (30, 31). Meanwhile, additional information on effectiveness and safety of Moderna, Sputnik V, and AstraZeneca vaccine on frail older adults is needed.
Limitations
There are several limitations to note in this study. Firstly, due to the rapid nature of the review, we are likely to have missed articles (41). Also, by restricting the eligibility criteria to Phase III studies in peer-reviewed journals and pre-prints of articles, vaccines such as Johnson & Johnson and Sinovac were not included in our review. Secondly, quality appraisal of the studies was not conducted in this rapid review due to time constraints, but it enabled capturing of pre-prints of relevant publications that have not been peer-reviewed. Third, attrition in the Delphi process might have led to response bias (42). Fourth, the criteria for consensus was subjective and thus open to bias (43). Lastly, experts’ opinions were also subjective and at risk of error (44).
Conclusion
Our rapid review of Phase III studies indicate that there is currently limited information available on the efficacy and safety of COVID-19 vaccination for frail older adults. Geriatricians from our Delphi panel indicated that frail or very old adults, except those with limited life expectancy, should be vaccinated as they are the most vulnerable group and at a higher risk of complications and death due to COVID-19. Lastly, the panel indicated that frail older adults should be included in future clinical trials of the vaccine.
References
World Health Organisation. Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Lim JP, Low KYH, Lin NJJ, et al. Predictors for development of critical illness amongst older adults with COVID-19: Beyond age to age-associated factors. Arch Gerontol Geriatr. 2021;94:104331. doi:https://doi.org/10.1016/j.archger.2020.104331
Yang Y, Luo K, Jiang Y, et al. The Impact of Frailty on COVID-19 Outcomes: A Systematic Review and Meta-analysis of 16 Cohort Studies. J Nutr Health Aging. 2021;25(5):702–709. doi:https://doi.org/10.1007/s12603-021-1611-9
National Population and Talent Division. Population in Brief 2020: Key Trends. Population in Brief 2020: Key Trends. https://www.population.gov.sg/media-centre/articles/population-in-brief-2020-key-trends
Vaingankar JA, Chong SA, Abdin E, et al. Prevalence of frailty and its association with sociodemographic and clinical characteristics, and resource utilization in a population of Singaporean older adults. Geriatr Gerontol Int. 2017;17(10):1444–1454. doi:https://doi.org/10.1111/ggi.12891
Berkeley Lovelace Jr. FDA approves second Covid vaccine for emergency use as it clears Moderna’s for U.S. distribution. CNBC. https://www.cnbc.com/2020/12/18/moderna-covid-vaccine-approved-fda-for-emergency-use.html. Published December 18, 2020.
Berkeley Lovelace Jr. FDA approves Pfizer’s Covid vaccine for emergency use as U.S. reaches pivotal moment in the pandemic. CNBC. December 11, 2020.
Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2020;384(5):403–416. doi:https://doi.org/10.1056/NEJMoa2035389
Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi:https://doi.org/10.1056/NEJMoa2034577
Torjesen I. Covid-19: Norway investigates 23 deaths in frail elderly patients after vaccination. BMJ. 2021;372. doi:https://doi.org/10.1136/bmj.n149
Rolland Y, Cesari M, Morley JE, Merchant R, Vellas B. COVID19 Vaccination in Frail People. Lots of Hope and Some Questions. J Nutr Health Aging. 2021;25(2):146–147. doi:https://doi.org/10.1007/s12603-021-1591-9
Veritas Health Innovation Ltd. Covidence. Veritas Health Innovation Ltd https://www.covidence.org/
CADTH. CADTH COVID-19 Search Strings. Accessed April 28, 2021. https://covid.cadth.ca/literature-searching-tools/cadth-covid-19-search-strings/
Niederberger M, Spranger J. Delphi Technique in Health Sciences: A Map. Front Public Health. 2020;8:457. doi:https://doi.org/10.3389/fpubh.2020.00457
Duchet-Niedziolka P, Launay O, Coutsinos Z, et al. Vaccination in adults with autoimmune disease and/or drug related immune deficiency: Results of the GEVACCIM Delphi survey. Vaccine. 2009;27(10):1523–1529. doi:https://doi.org/10.1016/j.vaccine.2009.01.003
Riva A, Barcella V, Benatti SV, et al. Vaccinations in patients with multiple sclerosis: A Delphi consensus statement. Mult Scler J. 2020;27(3):347–359. doi:https://doi.org/10.1177/1352458520952310
van Assen S, Agmon-Levin N, Elkayam O, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70(3):414. doi:https://doi.org/10.1136/ard.2010.137216
Nair R, Aggarwal R, Khanna D. Methods of Formal Consensus in Classification/ Diagnostic Criteria and Guideline Development. Semin Arthritis Rheum. 2011;41(2):95–105. doi:https://doi.org/10.1016/j.semarthrit.2010.12.001
Okoli C, Pawlowski SD. The Delphi method as a research tool: An example, design considerations and applications. Inf Manage. 2004;42(1):15–29. doi:https://doi.org/10.1016/j.im.2003.11.002
Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–409. doi:https://doi.org/10.1016/j.jclinepi.2013.12.002
Grant S, Booth M, Khodyakov D. Lack of preregistered analysis plans allows unacceptable data mining for and selective reporting of consensus in Delphi studies. J Clin Epidemiol. 2018;99:96–105. doi:https://doi.org/10.1016/j.jclinepi.2018.03.007
Akins RB, Tolson H, Cole BR. Stability of response characteristics of a Delphi panel: application of bootstrap data expansion. BMC Med Res Methodol. 2005;5:37. doi:https://doi.org/10.1186/1471-2288-5-37
Henderson EJ, Rubin GP. Development of a community-based model for respiratory care services. BMC Health Serv Res. 2012;12(1):193. doi:https://doi.org/10.1186/1472-6963-12-193
Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. The Lancet. Published online February 2, 2021. doi:https://doi.org/10.1016/S0140-6736(21)00234-8
Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. The Lancet. 2020;396(10267):1979–1993. doi:https://doi.org/10.1016/S0140-6736(20)32466-1
Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. The Lancet. 2021;397(10277):881–891. doi:https://doi.org/10.1016/S0140-6736(21)00432-3
Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. Published online February 24, 2021. doi:https://doi.org/10.1056/NEJMoa2101765
Bernal JL, Andrews N, Gower C, et al. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. Published online 2021. doi:https://doi.org/10.1101/2021.03.01.21252652
Vasileiou E, Simpson CR, Robertson C, et al. Effectiveness of First Dose of COVID-19 Vaccines Against Hospital Admissions in Scotland: National Prospective Cohort Study of 5.4 Million People. Published online 2021.
Salmerón Ríos S, Mas Romero M, Cortés Zamora EB, et al. Immunogenicity of the BNT162b2 vaccine in frail or disabled nursing home residents: COVID-A study. J Am Geriatr Soc. 2021;n/a(n/a). doi:https://doi.org/10.1111/jgs.17153
Parry HM, Tut G, Faustini S, et al. BNT162b2 Vaccination in People Over 80 Years of Age Induces Strong Humoral Immune Responses with Cross Neutralisation of P. 1 Brazilian Variant.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 2009;6(7):e1000097. doi:https://doi.org/10.1371/journal.pmed.1000097
Antonelli Incalzi R, Trevisan C, Del Signore S, et al. Are vaccines against COVID-19 tailored to the most vulnerable people? Vaccine. 2021;39(17):2325–2327. doi:https://doi.org/10.1016/j.vaccine.2021.03.066
Fried LP, Tangen CM, Walston J, et al. Frailty in Older Adults: Evidence for a Phenotype. J Gerontol Ser A. 2001;56(3):M146–M157. doi:https://doi.org/10.1093/gerona/56.3.M146
Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601–608. doi:https://doi.org/10.1007/s12603-012-0084-2
Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173(5):489. doi:https://doi.org/10.1503/cmaj.050051
Gustafson CE, Kim C, Weyand CM, Goronzy JJ. Influence of immune aging on vaccine responses. J Allergy Clin Immunol. 2020;145(5):1309–1321. doi:https://doi.org/10.1016/j.jaci.2020.03.017
Hyams C, Marlow R, Maseko Z, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: A test-negative, case-control study. Lancet Infect Dis. Published online June 23, 2021. doi:https://doi.org/10.1016/S1473-3099(21)00330-3
Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis Off Publ Infect Dis Soc Am. Published online April 27, 2021:ciab381. doi:https://doi.org/10.1093/cid/ciab381
Andrew MK, Schmader KE, Rockwood K, Clarke B, McElhaney JE. Considering Frailty in SARS-CoV-2 Vaccine Development: How Geriatricians Can Assist. Clin Interv Aging. 2021;16:731–738. doi:https://doi.org/10.2147/CIA.S295522
Grant MJ, Booth A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf Libr J. 2009;26(2):91–108. doi:https://doi.org/10.1111/j.1471-1842.2009.00848.x
Simoens S. Using the Delphi technique in economic evaluation: time to revisit the oracle? J Clin Pharm Ther. 2006;31(6):519–522. doi:https://doi.org/10.1111/j.1365-2710.2006.00780.x
Barrett D, Heale R. What are Delphi studies? Evid Based Nurs. 2020;23(3):68. doi:https://doi.org/10.1136/ebnurs-2020-103303
Evans D. Hierarchy of evidence: a framework for ranking evidence evaluating healthcare interventions. J Clin Nurs. 2003;12(1):77–84. doi:https://doi.org/10.1046/j.1365-2702.2003.00662.x
Acknowledgements
We would like to thank the Chapter of Geriatricians COVID-19 Vaccination Workgroup in Singapore for their input and participation in this study. In addition, we would like to express our sincere appreciation to Ms Yasmin Lynda Munro at the Nanyang Technological University, Lee Kong Chian School of Medicine Library, for her assistance in the review search strategy and the database searches.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest: None declared by all authors.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gao, J., Lun, P., Ding, Y.Y. et al. COVID-19 Vaccination for Frail Older Adults in Singapore — Rapid Evidence Summary and Delphi Consensus Statements. J Frailty Aging 11, 236–241 (2022). https://doi.org/10.14283/jfa.2022.12
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jfa.2022.12